ONBOARDING PROCEDURE CHECKLIST
|
|
|
- Helena Merritt
- 6 years ago
- Views:
Transcription
1 Northwestern RESEARCH OFFICE FOR RESEARCH INTEGRITY Research-Related PI ONBOARDING PROCEDURE CHECKLIST Before beginning this checklist you should first have a NetID. SPONSORED RESEARCH Are there awards transferring with you to Northwestern? Notify sponsors to begin transfer process. Contact your Northwestern Research Administrator throughout process. Complete appropriate relinquishment form depending on award and transferring institution, which will handle this step. For NIH Awards: PHS If an NIH Commons login already exists, contact the OSR-Information Team to swith your affiliation to Northwestern. With incoming faculty, the sponsored research office at both the outgoing institution and here at Northwestern will typically work together to determine which sponsored projects will be transferred, including personnel, lab equipment, data, biological samples, research animals, etc. The transfer (shipping, handling, insurance, etc.) is typically paid by the new institution. For NSF Awards: National Science Foundation (NSF) requires relinquishment information be submitted online via FastLane. CONTACT Office for Sponsored Research CHICAGO osr-chicago@ CONTACT Office for Sponsored Research EVANSTON osr-evanston@ Will material be transferred? If you are transferring material, begin the negotiation of a new Material Transfer Agreement (MTA). Complete a Material Transfer Agreement request for inbound materials through the Electronic Sponsored Projects Request (ESPR) system. When an MTA is received by the PI from an outside party in lieu of materials exchange or transfer, the agreement should be ed. CONTACT Material Transfer Agreement (MTA) officer mta@ Will data be transferred? If you are transferring data, you must establish a data use agreement (DUA) between the provider institution and Northwestern. Complete a Data Use Agreement request for inbound data through the Electronic Sponsored Projects Request (ESPR) system. DUA s are classified into two different categories: 1) Non-human subject data or completely de-identified human research participant data (as determined by NU s IRB office) 2) Human research participant data which includes Protected Health Information. This includes data which constitutes a Limited Data Set as defined by HIPAA. Transfers which fall into category 2 are subject to HIPAA regulations and may require IRB approval. Questions about IRB approval, guidelines and policies should be directed to irb@. CONTACT Material Transfer Agreement (MTA) officer CONTACT Office for Sponsored Research EVANSTON mta@ michael.rynties@
2 INSTITUTIONAL REVIEW BOARD (IRB) Does your work involve human research participants? Complete Northwestern Human Research Participant Protection Training. See the requirements and instructions for training. Provide list of All Human Research Participant Protocols that will be transferred to Northwestern. Provide contact information for the IRB Office that holds the current IRB approvals. List any required/requested special needs regarding IRB processes or trainings. Register with eirb. You must first have your NetID/Password from your department. CONTACT IRB Education Specialist INSTITUTIONAL ANIMAL CARE AND USE COMMITEE (IACUC) Does your work involve animal subjects? Submit an Animal Study Protocol (ASP). Submission and approval of a protocol must be completed prior to working with animals.* Complete IACUC training and enroll in the Occupational Health Safety Program (OHSP) online. Contact the IACUC office for assistance. Contact the IACUC office for guidance and assistance on protocol submission and review process. *You must have a NetID/password from your school/department to start your ASP submission into the system. IACUC staff will provide training to you. Submission and approval of a protocol must be completed prior to working with animals. All project investigators and research staff handling and caring for animals are required to take the basic IACUC on-line training and be enrolled in the Occupational Health and Safety Program (OHSP). In addition, anyone seeking facility access (CCM) or having contact with animals must be listed on an approved protocol. CONTACT the IACUC office acuc@ CENTER FOR COMPARATIVE MEDICINE Animal procurement, receiving, census and transfer Complete the New Investigator Questionnaire. Will you transfer animals to Northwestern? If YES: Complete the CCM Online Animal Shipment Form. You must have a NetID and password to login. Complete the Colony Move Spreadsheet. All sections must be filled out completely. Will you need to order animals once you arrive at Northwestern? If YES, login to the CCM website: To order animals from approved commercial vendors, select the Animal Procurement Form. It is critical that we know the number of cages/animals that the PI plans to work with and any other specialized care or equipment that they may require. If you do not have an approved protocol at the time of submission, leave the protocol number section blank but do provide a Northwestern departmental chartstring for billing until your protocol has been approved. Please let us know ASAP if any cages that you are shipping contain a pregnant mouse or pups. Your protocol must be approved and the appropriate funding linked PRIOR to ordering animals. To order animals from non-commercial vendors, go to the Animal Import Website. CONTACT La Tisha Jude Supervisor, Animal Procurement, Receiving and Census (PRC) l-jude@
3 CENTER FOR COMPARATIVE MEDICINE (CONTINUED) Training to work with animals Complete Facility Training before animals arrive at Northwestern. Contact CCM Quality and Training department to schedule a Lurie Orientation with Euthanasia Training for access to your animals. Review campus and species-specific checklists as a guide through training, the process of being added to an ASP, and being granted access to CCM. Research Checklists: - Rodent Checklist - Large Animal Checklist - Non-Human Primate Checklist *If you are a new researcher working with amphibians or fish, contact CCM Training Office. CONTACT Andrew Feeney, CCM Training Office CONTACT Matthew Taylor, CCM Training Manager andrew.feeney@northwestern. edu matthew.taylor1@ RESEARCH SAFETY Is the PI involved in laboratory-based research? Laboratory-based research involves the use of dedicated facilities for experimentation or measurement supplied with one or more utilities such as ventilation, storage, and plumbing. Register for Northwestern Integrated Safety Information System (NISIS) Orientation at ors-operations@. Once you register, you will receive an notification of your access to the system. In NSIS, register your lab workers and assign safety training. Register lab locations and work involving hazardous energies, lasers, x-ray, and hazardous materials within 30 days. The subject line of your to register should read Register for an Orientation Session and include your name, department, anticipated date of arrival, contact phone number and/or address in the message. Hazardous materials include any biological, chemical, or radiological material that is potentially harmful to individuals, public health, or the environment if not managed properly. CONTACT Michael Blaney, Executive Director michael.blayney@ NORTHWESTERN CONFLICT OF INTEREST (COI) Familiarize yourself with Northwestern s Policy on Conflict of Interest in Research and Policy on Conflict of Interest and Conflict of Commitment. -One page guide for research-related COI process -Quick tips for COI training and disclosure requirements Undergo conflict of interest training online in edisclosure. Complete and submit a disclosure in edisclosure. CONTACT Julia Campbell, COI Director juliacampbell@
4 EXPORT CONTROLS Review and familiarize yourself with Northwestern s Policy on Export Controls Compliance. Confirm that you are not engaged in any research projects that are specifically subject to the export control regulations, e.g. the ITAR, EAR, or any projects that have restrictions upon publication or foreign national participation. For more on Northwestern s Export Controls Compliance responsibilities and additional regulation resources and links, visit the OECC website. CONTACT Lane Campbell, Director lcampbell@ CORE FACILITIES If you want to purchase equipment or a data acquisition system costing $300K or more, contact Philip Hockberger. There is no form, but there is an internal vetting process for external grant applications. Office for Research provides administrative assistance regarding placement of instruments, technical support, service contracts, and UPS requirements. CONTACT Philip Hockberger, Assistant Vice President p-hockberger@ INNOVATIONS AND NEW VENTURES OFFICE (INVO) If your work requires a proof-of-concept to validate a product concept, contact INVO. If your work might lead to a potentially patentable invention, please fill out an Invention Disclosure Form (available at the INVO website), and submit it to INVO BEFORE publication or another public disclosure (e.g., conference presentation or abstract) occurs. If you are interested in start-ups to advance innovative concepts, call INVO. If you have previously filed patents, have been involved in a start-up company, or intend to utilize pre-existing IP in your work, contact INVO. A number of approaches are evolving at Northwesern to help define product opportunities and fund proof-of-concept studies in the physical and life sciences. INVO works closely with faculty to shape invention disclosures and to secure intellectual property. INVO is at the center of a network of resources to introduce faculty and students to the world of start-up companies and to facilitiate their participation. Please contact INVO to facilitate a conversation between your previous institution and Northwestern. CONTACT INVO Office NORTHWESTERN CLINICAL AND TRANSLATIONAL SCIENCES INSTITUTE (NUCATS) Meet to discuss clinical research resources and services available to PIs. The PI can meet with the Clinical Research Navigator in NUCATS to learn about resources and services that are available. CONTACT NUCATS Office ACCOUNTING SERVICES FOR RESEARCH AND SPONSORED PROGRAMS (ASRSP) A Final Financial Report (FFR) should be sent from your previous institution to your new Research Administrator at Northwestern. Contact your ASRSP Grant and Contract Financial Administrator for assistance. This will allow ASRSP to track the transfer of carry-over from your previous institution. Visit the ASRSP website to assist with the monitoring of sponsored funds. CONTACT Karen Spina, Chicago kspina@ CONTACT Howard Ventura, Evanston h-ventura@
5 EFFORT REPORTING / COST STUDIES For policies and procedures regarding effort certification as well as guidance on charging sponsored projects, visit the Cost Studies website. See the Introduction to Effort Reporting mini-course online. CONTACT Jennifer Mitchell Associate Executive Director for Research Financial Operations jennifer-mitchell@ OFFICE OF RESEARCH DEVELOPMENT Meet to discuss the federal funding research landscape and learn about ORD services available to PIs Contact ORD to setup a meeting to learn about limited submissions, federal funding opportunities, proposal development support, and discuss how to grow and diversify your federal funding portfolio. CONTACT Nicole Moore, Director nicole.moore@ OnBoarding_Doc082417
The Office for Sponsored Research (OSR): An Overview. Office for Sponsored Research
 The Office for Sponsored Research (OSR): An Overview Office for Sponsored Research OSR Mission The Office for Sponsored Research assists investigators in proposing and managing sponsored programs in support
The Office for Sponsored Research (OSR): An Overview Office for Sponsored Research OSR Mission The Office for Sponsored Research assists investigators in proposing and managing sponsored programs in support
NURAP: COI Update. New edisclosure System. January 21, 2016 Julia Campbell Director, Conflict of Interest
 NURAP: COI Update New edisclosure System January 21, 2016 Julia Campbell Director, Conflict of Interest Topics for Discussion Overview of new system Impacts on research-related processes Timeline Training
NURAP: COI Update New edisclosure System January 21, 2016 Julia Campbell Director, Conflict of Interest Topics for Discussion Overview of new system Impacts on research-related processes Timeline Training
Expense Review Process and Internal Controls for Award Closeout. Semi-annual Networking Event February 2017
 ACCOUNTING SERVICES FOR RESEARCH AND SPONSORED PROGRAMS (ASRSP) Expense Review Process and Internal Controls for Award Closeout Semi-annual Networking Event February 2017 Kathy Mustea, Assistant Director
ACCOUNTING SERVICES FOR RESEARCH AND SPONSORED PROGRAMS (ASRSP) Expense Review Process and Internal Controls for Award Closeout Semi-annual Networking Event February 2017 Kathy Mustea, Assistant Director
Roles and Responsibilities of Students and Adults
 Roles and Responsibilities of Students and Adults The Student Researcher The student researcher is responsible for all aspects of the research project including enlisting the aid of any required supervisory
Roles and Responsibilities of Students and Adults The Student Researcher The student researcher is responsible for all aspects of the research project including enlisting the aid of any required supervisory
Conflict of Interest/Commitment
 Conflict of Interest/Commitment Conflict of Interest Office (NU) Kate Booth Senior Compliance Specialist Defining a Conflict of Interest A situation where an individual s external financial interests may
Conflict of Interest/Commitment Conflict of Interest Office (NU) Kate Booth Senior Compliance Specialist Defining a Conflict of Interest A situation where an individual s external financial interests may
Ask the Experts Panel
 Ask the Experts Panel Compliance in Research Colleen Fritsche, Assistant Director of Office of Animal Care and Use Cassie Myers, Deputy Director of Office of Human Research Ethics Chris Nelson, Director
Ask the Experts Panel Compliance in Research Colleen Fritsche, Assistant Director of Office of Animal Care and Use Cassie Myers, Deputy Director of Office of Human Research Ethics Chris Nelson, Director
ALL PROJECTS. Eligibility/Limitations 1. Each Intel ISEF-affiliated fair may send the number of projects provided by their affiliation agreement.
 ALL PROJECTS Ethics Statement Scientific fraud and misconduct are not condoned at any level of research or competition. This includes plagiarism, forgery, use or presentation of other researcher s work
ALL PROJECTS Ethics Statement Scientific fraud and misconduct are not condoned at any level of research or competition. This includes plagiarism, forgery, use or presentation of other researcher s work
NUMBER: / /2009
 Research Compliance ISSUED: 11/2002 REV. D: 11/2009 REV. A: REV. B: REV. C: 10/2005 7/2007 7/2008 11/2006 REV. E: 8/2016 PAGE 1 OF 5 IACUC 2.1 IACUC Members Initial Training All new IACUC members receive
Research Compliance ISSUED: 11/2002 REV. D: 11/2009 REV. A: REV. B: REV. C: 10/2005 7/2007 7/2008 11/2006 REV. E: 8/2016 PAGE 1 OF 5 IACUC 2.1 IACUC Members Initial Training All new IACUC members receive
Purpose. Regulatory Background. Scope. Responsibility. Princeton University Institutional Animal Care and Use Committee Policy
 IACUC Number: 201 Version Number: 2.0 Approval Date: November 20, 2014 Effective Date: November 20, 2014 Title: Education and Training of Animal Care and Use Personnel Purpose This policy provides a standard
IACUC Number: 201 Version Number: 2.0 Approval Date: November 20, 2014 Effective Date: November 20, 2014 Title: Education and Training of Animal Care and Use Personnel Purpose This policy provides a standard
Roles and Responsibilities of Students and Adults
 Roles and Responsibilities of Students and Adults 1) The Student Researcher(s) The student researcher is responsible for all aspects of the research project including enlisting the aid of any needed supervisory
Roles and Responsibilities of Students and Adults 1) The Student Researcher(s) The student researcher is responsible for all aspects of the research project including enlisting the aid of any needed supervisory
Best Practices for Sponsored Project Transfers
 Office of Sponsored Programs (OSP) Roundtable Best Practices for Sponsored Project Transfers Bella DiFranzo, Grant and Contract Officer, Office of Sponsored Programs Rajni Aneja, Sr. Grant and Contract
Office of Sponsored Programs (OSP) Roundtable Best Practices for Sponsored Project Transfers Bella DiFranzo, Grant and Contract Officer, Office of Sponsored Programs Rajni Aneja, Sr. Grant and Contract
UTHSCSA LAB TRANSFER INFORMATION
 UTHSCSA LAB TRANSFER INFORMATION The information provided below is a list of offices and contacts that are needed for various approvals to move a lab to UTHSCSA. Animal Transfer to UTHSCSA http://research.uthscsa.edu/lar/
UTHSCSA LAB TRANSFER INFORMATION The information provided below is a list of offices and contacts that are needed for various approvals to move a lab to UTHSCSA. Animal Transfer to UTHSCSA http://research.uthscsa.edu/lar/
Proposal Summary Example
 Example 04/2012 https://umces.cayuse424.com:8443/319/showproposalpage.do?subsessionid=0&url=/... Proposal Summary Proposal Number AL2011-999NSF Proposal Status: Incomplete Sponsor Deadline 01/01/2012 Submission
Example 04/2012 https://umces.cayuse424.com:8443/319/showproposalpage.do?subsessionid=0&url=/... Proposal Summary Proposal Number AL2011-999NSF Proposal Status: Incomplete Sponsor Deadline 01/01/2012 Submission
Grants Module. Create and Submit a Funding Proposal for Non System to System Submissions
 Grants Module Create and Submit a Funding Proposal for Non System to System Submissions Summary of Major Changes The following directions are a combination of all steps in the Funding Proposal submission
Grants Module Create and Submit a Funding Proposal for Non System to System Submissions Summary of Major Changes The following directions are a combination of all steps in the Funding Proposal submission
RESIN (Research Support Information Network) Presented by: Office of the Vice Chancellor for Research Date: June 24, 2011
 RESIN (Research Support Information Network) Presented by: Office of the Vice Chancellor for Research Date: June 24, 2011 Agenda Updates & Timely Information from Research Support: Office of the VCR RSC
RESIN (Research Support Information Network) Presented by: Office of the Vice Chancellor for Research Date: June 24, 2011 Agenda Updates & Timely Information from Research Support: Office of the VCR RSC
Sponsored Program Administration
 Sponsored Program Administration March, 2018 research.uconn.edu AGENDA OVPR/SPS News and Information IPR Form FCOI SPS Procedures for Fund Set Up Banner NIH News and Information Federal Executive Pay Scale
Sponsored Program Administration March, 2018 research.uconn.edu AGENDA OVPR/SPS News and Information IPR Form FCOI SPS Procedures for Fund Set Up Banner NIH News and Information Federal Executive Pay Scale
THINKING OF MOVING: A GUIDE FOR TRANSFERRING AN NIH GRANT TO A NEW INSTITUTION
 THINKING OF MOVING: A GUIDE FOR TRANSFERRING AN NIH GRANT TO A NEW INSTITUTION It is not unusual for NIH funded Principal Investigators (PIs) to move from one institution to another. We fully expect that
THINKING OF MOVING: A GUIDE FOR TRANSFERRING AN NIH GRANT TO A NEW INSTITUTION It is not unusual for NIH funded Principal Investigators (PIs) to move from one institution to another. We fully expect that
PROPOSAL ROUTING FORM INSTRUCTIONS Dartmouth College/Dartmouth Hitchcock Medical Center
 PROPOSAL ROUTING FORM INSTRUCTIONS Dartmouth College/Dartmouth Hitchcock Medical Center INTRODUCTION This routing form must be completed for all proposals submitted to external funding entities. The form
PROPOSAL ROUTING FORM INSTRUCTIONS Dartmouth College/Dartmouth Hitchcock Medical Center INTRODUCTION This routing form must be completed for all proposals submitted to external funding entities. The form
OSR Monthly Meeting. August 16, 2018 (Chicago) August 21, 2018 (Evanston)
 OSR Monthly Meeting August 16, 2018 (Chicago) August 21, 2018 (Evanston) Agenda OSR Announcements/Staffing Updates ESPR Replacement Project Update Year-end Procedures NSF Updates NIH Biosketch Reminder
OSR Monthly Meeting August 16, 2018 (Chicago) August 21, 2018 (Evanston) Agenda OSR Announcements/Staffing Updates ESPR Replacement Project Update Year-end Procedures NSF Updates NIH Biosketch Reminder
Guidance on Grant Award Transfers
 University of the Pacific Office of Sponsored Programs Guidance on Grant Award Transfers It is not unusual for funded Principal Investigators (PIs) to move from one institution to another. However, The
University of the Pacific Office of Sponsored Programs Guidance on Grant Award Transfers It is not unusual for funded Principal Investigators (PIs) to move from one institution to another. However, The
UNM Cayuse SP User Guide
 UNM Cayuse SP User Guide Cayuse Research Suite is used by the UNM Office of Sponsored Projects to manage proposal submissions to funding agencies and track awards for the Main and Branch campuses. This
UNM Cayuse SP User Guide Cayuse Research Suite is used by the UNM Office of Sponsored Projects to manage proposal submissions to funding agencies and track awards for the Main and Branch campuses. This
Administrative Burden of Research Compliance
 Administrative Burden of Research Compliance Measuring and Minimizing David L. Wynes, Ph.D. Vice President for Research Administration Emory University 1 FDP Faculty Burden Survey (X2) PIs estimated that
Administrative Burden of Research Compliance Measuring and Minimizing David L. Wynes, Ph.D. Vice President for Research Administration Emory University 1 FDP Faculty Burden Survey (X2) PIs estimated that
Orientation to Research & Sponsored Projects Session II: Preparing Your Proposal. September 15, 2010
 Orientation to Research & Sponsored Projects Session II: Preparing Your Proposal September 15, 2010 1 Welcome & Introductions Agenda Ann Q. Gates, Associate Vice President for Research Overview: Roles
Orientation to Research & Sponsored Projects Session II: Preparing Your Proposal September 15, 2010 1 Welcome & Introductions Agenda Ann Q. Gates, Associate Vice President for Research Overview: Roles
The Lifecycle of a Sponsored Project
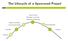 The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
Research & Funding A Step-by-Step Guide
 Research & Funding A Step-by-Step Guide These steps outline what is necessary to complete a research project from start to finish. The order of the steps is required for any investigator; however, which
Research & Funding A Step-by-Step Guide These steps outline what is necessary to complete a research project from start to finish. The order of the steps is required for any investigator; however, which
Welcome to A Beginner s Guide to Sponsored Project Solicitations. This is one of the introductory mini courses in Northwestern s Sponsored Project
 Welcome to A Beginner s Guide to Sponsored Project Solicitations. This is one of the introductory mini courses in Northwestern s Sponsored Project Online Training series. 1 If you have previously joined
Welcome to A Beginner s Guide to Sponsored Project Solicitations. This is one of the introductory mini courses in Northwestern s Sponsored Project Online Training series. 1 If you have previously joined
Office of Research Compliance
 Office of Research Compliance Office of Research Compliance Debra Keppler Director Responsible for providing programs to address: Responsible conduct of research training (RCR) Export control compliance
Office of Research Compliance Office of Research Compliance Debra Keppler Director Responsible for providing programs to address: Responsible conduct of research training (RCR) Export control compliance
Spartan RAN. Research Administrators Network Biannual Meeting April 23 rd, Before we get started, please follow the instructions on your table!
 Spartan RAN Research Administrators Network Biannual Meeting April 23 rd, 2015 Before we get started, please follow the instructions on your table! We re Going Green in 2015, so paper handouts will not
Spartan RAN Research Administrators Network Biannual Meeting April 23 rd, 2015 Before we get started, please follow the instructions on your table! We re Going Green in 2015, so paper handouts will not
PEER (PAPERLESS ENVIRONMENT FOR ELECTRONIC REVIEW)
 PEER (PAPERLESS ENVIRONMENT FOR ELECTRONIC REVIEW) 1 What is PEER? PEER is a web portal maintained by Vanderbilt University which handles internal processing of grant related requests and contract submission
PEER (PAPERLESS ENVIRONMENT FOR ELECTRONIC REVIEW) 1 What is PEER? PEER is a web portal maintained by Vanderbilt University which handles internal processing of grant related requests and contract submission
Proposal Preparation and Submission February 2018
 Proposal Preparation and Submission February 2018 Introductions We are. Chris Dye-Hixenbaugh, C&G Officer, Proposals Brooke Herevia, C&G Analyst, Proposals Shanna Nation Jose, C&G Analyst, Proposals Kassie
Proposal Preparation and Submission February 2018 Introductions We are. Chris Dye-Hixenbaugh, C&G Officer, Proposals Brooke Herevia, C&G Analyst, Proposals Shanna Nation Jose, C&G Analyst, Proposals Kassie
Research and Economic Development at UC Riverside
 Research and Economic Development at UC Riverside Michael Pazzani Vice Chancellor for Research and Economic Development pazzani@ucr.edu http://research.ucr.edu Making an impact Overview Do great research
Research and Economic Development at UC Riverside Michael Pazzani Vice Chancellor for Research and Economic Development pazzani@ucr.edu http://research.ucr.edu Making an impact Overview Do great research
Loyola University Chicago Health Sciences Division Maywood, IL. Human Subject Research Project Start-Up Guide
 Loyola University Chicago Health Sciences Division Maywood, IL Human Subject Research Project Start-Up Guide This Start-Up Guide is intended to guide you through the process of designing a research project
Loyola University Chicago Health Sciences Division Maywood, IL Human Subject Research Project Start-Up Guide This Start-Up Guide is intended to guide you through the process of designing a research project
INTRODUCTION TO RESEARCH ADMINISTRATION. Office of Grants and Contracts Administration August 2015
 INTRODUCTION TO RESEARCH ADMINISTRATION Office of Grants and Contracts Administration August 2015 THE RESEARCH VESSEL SIKULIAQ FUNDED BY THE NATIONAL SCIENCE FOUNDATION Objectives To understand the UAF
INTRODUCTION TO RESEARCH ADMINISTRATION Office of Grants and Contracts Administration August 2015 THE RESEARCH VESSEL SIKULIAQ FUNDED BY THE NATIONAL SCIENCE FOUNDATION Objectives To understand the UAF
R.A.I.S.E. Research Administration Information Sharing & Education - Cohort Series
 R.A.I.S.E. Research Administration Information Sharing & Education - Cohort Series The R.A.I.S.E. Series is a cohort style training program led by Carnegie Mellon s own subject matter experts who will
R.A.I.S.E. Research Administration Information Sharing & Education - Cohort Series The R.A.I.S.E. Series is a cohort style training program led by Carnegie Mellon s own subject matter experts who will
Narration: Welcome to the Anatomy of an Administrative Shell mini course.
 Welcome to the Anatomy of an Administrative Shell mini course. 1 If you have previously joined us for other sponsored project mini courses, you will be familiar with the Sponsored Project Life Cycle. In
Welcome to the Anatomy of an Administrative Shell mini course. 1 If you have previously joined us for other sponsored project mini courses, you will be familiar with the Sponsored Project Life Cycle. In
Strategic Plan wmich.edu/research
 wmich.edu/research INTRODUCTION Western Michigan University is learner centered, discovery driven, and globally engaged. It became Michigan s fourth public university in 1957 and today offers 147 bachelor
wmich.edu/research INTRODUCTION Western Michigan University is learner centered, discovery driven, and globally engaged. It became Michigan s fourth public university in 1957 and today offers 147 bachelor
PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR CONTACT INFORMATION Prefix: First Name: Middle Name: Last Name: Suffix:
 Proposal Summary Proposal Number Sponsor Deadline Proposal Status: Submission Method: Submission Type: Pre-application Application Changed/Corrected INVESTIGATOR DATA PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR
Proposal Summary Proposal Number Sponsor Deadline Proposal Status: Submission Method: Submission Type: Pre-application Application Changed/Corrected INVESTIGATOR DATA PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR
Research Support Proposal APPLICATION INSTRUCTIONS
 Research Support Proposal APPLICATION INSTRUCTIONS Research Project Letters of Intent are currently accepted on a ROLLING SUBMISSION basis. Applications must be submitted electronically in WORD format*
Research Support Proposal APPLICATION INSTRUCTIONS Research Project Letters of Intent are currently accepted on a ROLLING SUBMISSION basis. Applications must be submitted electronically in WORD format*
3 rd Annual Symposium for Research Administrators
 3 rd Annual Symposium for Research Administrators The UNC-CH Research IT Landscape An overview of the systems that support the research enterprise John Stephenson john@unc.edu July 29, 2016 Research Administration
3 rd Annual Symposium for Research Administrators The UNC-CH Research IT Landscape An overview of the systems that support the research enterprise John Stephenson john@unc.edu July 29, 2016 Research Administration
Acquisition, Management, Sharing, and Ownership of Data
 Acquisition, Management, Sharing, and Ownership of Data Responsible Conduct of Research Training F R A N K V A N B R E U K E L E N U N I V E R S I T Y O F N E V A D A L A S V E G A S P R E S E N T E D
Acquisition, Management, Sharing, and Ownership of Data Responsible Conduct of Research Training F R A N K V A N B R E U K E L E N U N I V E R S I T Y O F N E V A D A L A S V E G A S P R E S E N T E D
Post-Doctoral Researcher - Researcher Startup Tool 1 of 8
 Post-Doctoral Researcher - Researcher Startup Tool 1 of 8 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR and
Post-Doctoral Researcher - Researcher Startup Tool 1 of 8 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR and
Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan
 Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan TABLE OF CONTENTS GRASP COMPLIANCE PROGRAM Policy Applicability Components Administration GRASP COMPLIANCE PLAN Introduction
Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan TABLE OF CONTENTS GRASP COMPLIANCE PROGRAM Policy Applicability Components Administration GRASP COMPLIANCE PLAN Introduction
IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/ :19 PM
 IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/2018 12:19 PM Topics Covered Class Objective:. To become more familiar with the IRES
IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/2018 12:19 PM Topics Covered Class Objective:. To become more familiar with the IRES
Proposal Submission, Review and Acceptance
 Proposal Submission, Review and Acceptance October 2015 Peggy Bowe OGRD is now OROS Office of Research Operations and Support Recording date of this workshop is October 23, 2015 Some of the rules and procedures
Proposal Submission, Review and Acceptance October 2015 Peggy Bowe OGRD is now OROS Office of Research Operations and Support Recording date of this workshop is October 23, 2015 Some of the rules and procedures
Principal Investigator/Co-Investigator - Researcher Startup Tool 1 of 9
 Principal Investigator/Co-Investigator - Researcher Startup Tool 1 of 9 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to
Principal Investigator/Co-Investigator - Researcher Startup Tool 1 of 9 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to
UMCES CAYUSE 424 Training 7/21/2010 1
 UMCES CAYUSE 424 Training 7/21/2010 1 A new routing process... UMCES is moving toward using the CAYUSE424 platform for all proposal submissions. - Goal: July 1, 2010. CAYUSE is a system to system standard
UMCES CAYUSE 424 Training 7/21/2010 1 A new routing process... UMCES is moving toward using the CAYUSE424 platform for all proposal submissions. - Goal: July 1, 2010. CAYUSE is a system to system standard
Management and Administration of Sponsored Projects
 Augusta University Policy Library Management and Administration of Sponsored Projects Policy Owner: Sponsored Program Administration POLICY STATEMENT This policy is to guide Principal Investigators, Investigators,
Augusta University Policy Library Management and Administration of Sponsored Projects Policy Owner: Sponsored Program Administration POLICY STATEMENT This policy is to guide Principal Investigators, Investigators,
CAYUSE Research Suite
 CAYUSE Research Suite Internal Clearance & Submission Process Principal Investigator/Program Director Cayuse SP is used by ORSP to process Internal Clearance and when applicable to submit a proposal for
CAYUSE Research Suite Internal Clearance & Submission Process Principal Investigator/Program Director Cayuse SP is used by ORSP to process Internal Clearance and when applicable to submit a proposal for
Navigating Grant Resources. 10/3/2018 Dr. Susan Fell
 Navigating Grant Resources 10/3/2018 Dr. Susan Fell sfell@fsu.edu 850-645-2172 Sponsored Research Administration https://www.research.fsu.edu/ Applying for a Grant Opportunity Does this require sign-off
Navigating Grant Resources 10/3/2018 Dr. Susan Fell sfell@fsu.edu 850-645-2172 Sponsored Research Administration https://www.research.fsu.edu/ Applying for a Grant Opportunity Does this require sign-off
Moving your Research to Market
 Moving your Research to Market Seize the opportunity Sacha Patera, PhD Associate Director of Corporate Relations Northwestern University, Evanston & Chicago IL 2011 NSF ADVANCE Meeting 10 Years of Broadening
Moving your Research to Market Seize the opportunity Sacha Patera, PhD Associate Director of Corporate Relations Northwestern University, Evanston & Chicago IL 2011 NSF ADVANCE Meeting 10 Years of Broadening
ANIMAL CARE & USE MANUAL
 ANIMAL CARE & USE MANUAL Missouri State University (MSU) and its Institutional Animal Care & Use Committee (IACUC) is committed to an animal care and use program of the highest quality. Missouri State
ANIMAL CARE & USE MANUAL Missouri State University (MSU) and its Institutional Animal Care & Use Committee (IACUC) is committed to an animal care and use program of the highest quality. Missouri State
RESEARCH ADMINISTRATION STANDARD OPERATING PROCEDURES (SOPS)
 RESEARCH ADMINISTRATION STANDARD OPERATING PROCEDURES (SOPS) Award Obligation Setup (AOS) University Prior Approval Form (UPAF) Agreements Reviewed & Approved by RA Northeastern University Office Of Research
RESEARCH ADMINISTRATION STANDARD OPERATING PROCEDURES (SOPS) Award Obligation Setup (AOS) University Prior Approval Form (UPAF) Agreements Reviewed & Approved by RA Northeastern University Office Of Research
SOCIOLOGY GRADUATE STUDENT WORKSHOP
 SOCIOLOGY GRADUATE STUDENT WORKSHOP Weinberg Research Administration May 9, 2016 http://www.weinberg.northwestern.edu/research/administration/index.html December, 2013 Research Administration in the Weinberg
SOCIOLOGY GRADUATE STUDENT WORKSHOP Weinberg Research Administration May 9, 2016 http://www.weinberg.northwestern.edu/research/administration/index.html December, 2013 Research Administration in the Weinberg
Electronic Research Administration (era) March 8, 2018
 Electronic Research Administration (era) March 8, 2018 Introductions We are. Terry Duperron, era Analyst Marlene Mooshian, Proposal Analyst Kassie Obelleiro, Training Officer Jinger Snyder, Award Analyst
Electronic Research Administration (era) March 8, 2018 Introductions We are. Terry Duperron, era Analyst Marlene Mooshian, Proposal Analyst Kassie Obelleiro, Training Officer Jinger Snyder, Award Analyst
HARVARD UNIVERSITY MINORS IN LABS POLICY STATEMENT
 Policy Title: Minors in Labs Responsible Office: EH&S Effective Date: January 1, 2016 Revision Date: December 3, 2015 POLICY STATEMENT Harvard is committed to fostering a safe environment for minors and
Policy Title: Minors in Labs Responsible Office: EH&S Effective Date: January 1, 2016 Revision Date: December 3, 2015 POLICY STATEMENT Harvard is committed to fostering a safe environment for minors and
(Insert additional Principal Investigators in the Comments section.) Co-Investigator Data Investigators Employee # School
 University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
UNC Lineberger Developmental Funding Program. Proposal Due Dates: 5:00pm March 15 and September 15
 Proposal Due Dates: 5:00pm March 15 and September 15 Letter of Intent for Tier 3: Multi-Project Awards due three weeks before due date The UNC Lineberger Developmental Funding Program is intended to support
Proposal Due Dates: 5:00pm March 15 and September 15 Letter of Intent for Tier 3: Multi-Project Awards due three weeks before due date The UNC Lineberger Developmental Funding Program is intended to support
Colorado State University Export Compliance Questionnaire I-I29 Petition for a Non-Immigrant Worker
 Colorado State University Export Compliance Questionnaire I-I29 Petition for a Non-Immigrant Worker Date: Information about current/proposed employee: Name: Country of Citizenship: Non-immigrant status
Colorado State University Export Compliance Questionnaire I-I29 Petition for a Non-Immigrant Worker Date: Information about current/proposed employee: Name: Country of Citizenship: Non-immigrant status
USCERA University of South Carolina Electronic Research Administration User Guide
 USCERA University of South Carolina Electronic Research Administration User Guide https://sam.research.sc.edu/uscera For more information contact: Sponsored Awards Management (803)777-7093 Developed September
USCERA University of South Carolina Electronic Research Administration User Guide https://sam.research.sc.edu/uscera For more information contact: Sponsored Awards Management (803)777-7093 Developed September
Effectively Managing and Monitoring Controlled Substances in Research
 Effectively Managing and Monitoring Controlled Substances in Research Emmelyn Kim, MA, MPH, CCRA, CHRC AVP, Research Compliance & Privacy Officer Ji Eun Kim, PhD, RPh Research Pharmacist The Office of
Effectively Managing and Monitoring Controlled Substances in Research Emmelyn Kim, MA, MPH, CCRA, CHRC AVP, Research Compliance & Privacy Officer Ji Eun Kim, PhD, RPh Research Pharmacist The Office of
Topics 5/16/2017. Effectively Managing and Monitoring Controlled Substances in Research
 Effectively Managing and Monitoring Controlled Substances in Research Emmelyn Kim, MA, MPH, CCRA, CHRC AVP, Research Compliance & Privacy Officer Ji Eun Kim, PhD, RPh Research Pharmacist The Office of
Effectively Managing and Monitoring Controlled Substances in Research Emmelyn Kim, MA, MPH, CCRA, CHRC AVP, Research Compliance & Privacy Officer Ji Eun Kim, PhD, RPh Research Pharmacist The Office of
Operational Guidelines for Scientific Review Committees (SRC) and Institutional Review Boards (IRB)
 Operational Guidelines for Scientific Review Committees (SRC) and Institutional Review Boards (IRB) For specific rules, please refer to: International Rules for Precollege Science Research: Guidelines
Operational Guidelines for Scientific Review Committees (SRC) and Institutional Review Boards (IRB) For specific rules, please refer to: International Rules for Precollege Science Research: Guidelines
Procedure for Addressing PHS Animal Protocol-Proposal Congruency Requirements at the UMass Medical School
 Section I. Introduction A. In recent years, the NIH Office of Laboratory Animal Welfare has increasingly emphasized the importance of NIH-funded institutions proactively ensuring that their Investigators
Section I. Introduction A. In recent years, the NIH Office of Laboratory Animal Welfare has increasingly emphasized the importance of NIH-funded institutions proactively ensuring that their Investigators
WENTZ RESEARCH GRANT PRESS START
 WENTZ RESEARCH GRANT PRESS START INFORMATION SESSION TOPICS Applying for a Wentz Research Grant Finding a Faculty Mentor Writing a Research Proposal Handling Ethical Compliance APPLYING FOR A WENTZ RESEARCH
WENTZ RESEARCH GRANT PRESS START INFORMATION SESSION TOPICS Applying for a Wentz Research Grant Finding a Faculty Mentor Writing a Research Proposal Handling Ethical Compliance APPLYING FOR A WENTZ RESEARCH
Office of Research Development Internal Funding Arts and Humanities Research Award
 Eligibility, regulations, and online research application instructions for the Arts and Humanities Research Award program PROGRAM DESCRIPTION The Arts and Humanities Research Grant is a pilot funding program
Eligibility, regulations, and online research application instructions for the Arts and Humanities Research Award program PROGRAM DESCRIPTION The Arts and Humanities Research Grant is a pilot funding program
Space Activity Coding and F&A Rates
 Space Activity Coding and F&A Rates Contact for Space Activity Coding and F&A Proposal: Jennifer Mitchell Office of Cost Studies jmitchell@northwestern.edu 847.467.2473 Sophia Gabay Office of Cost Studies
Space Activity Coding and F&A Rates Contact for Space Activity Coding and F&A Proposal: Jennifer Mitchell Office of Cost Studies jmitchell@northwestern.edu 847.467.2473 Sophia Gabay Office of Cost Studies
Minimum Proposal Requirements for Routing
 Minimum Proposal Requirements for Routing This QuickCard contains the minimum requirements for routing a proposal for approval in Coeus. Review the sponsor s solicitation for specific requirements and
Minimum Proposal Requirements for Routing This QuickCard contains the minimum requirements for routing a proposal for approval in Coeus. Review the sponsor s solicitation for specific requirements and
Clinical Research Coordinator - Researcher Startup Tool 1 of 7
 Clinical Research Coordinator - Researcher Startup Tool 1 of 7 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR
Clinical Research Coordinator - Researcher Startup Tool 1 of 7 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR
SJSU Research Foundation
 SJSU Research Foundation Grant Proposal Development Taking a Bite Out of Forms Sponsored Programs Workshop November 19, 2015 Overview of Workshop Why We Have Forms Forms from OSP Forms from Human Resources
SJSU Research Foundation Grant Proposal Development Taking a Bite Out of Forms Sponsored Programs Workshop November 19, 2015 Overview of Workshop Why We Have Forms Forms from OSP Forms from Human Resources
Grant/Sponsor Related Systems. Department and OSP Perspectives on ERA
 Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
A program for standardized training in rodent handling at a large academic institution
 A program for standardized training in rodent handling at a large academic institution Tracy Heenan, DVM, CPIA In large, decentralized institutions, providing consistent training to the substantial numbers
A program for standardized training in rodent handling at a large academic institution Tracy Heenan, DVM, CPIA In large, decentralized institutions, providing consistent training to the substantial numbers
Clinical Research Coordinator - Researcher Startup Tool 1 of 7
 Clinical Research Coordinator - Researcher Startup Tool 1 of 7 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR
Clinical Research Coordinator - Researcher Startup Tool 1 of 7 Mount Sinai Hospital Employees: ITHelpDesk@mountsinai.org 212-241-4357 Sinai Central Account Sinai Central is the system used to manage HR
KickStart Venture Services Commercialization Award Program
 KickStart Venture Services Commercialization Award Program Request for Applications Description The KickStart Venture Services Commercialization Award Program will provide up to $50K in non-dilutive funding
KickStart Venture Services Commercialization Award Program Request for Applications Description The KickStart Venture Services Commercialization Award Program will provide up to $50K in non-dilutive funding
SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME
 SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME OVPR/SPS News and Information Technology Commercialization Services Andrew Zehner 2017 Individual Financial Disclosure in Research Internal Routing
SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME OVPR/SPS News and Information Technology Commercialization Services Andrew Zehner 2017 Individual Financial Disclosure in Research Internal Routing
Ins and Outs of SBIR Grants and Contracts. A Grants Manager Perspective
 Ins and Outs of SBIR Grants and Contracts A Grants Manager Perspective Andre D. Walker Outline Differences Between a Grant and Contract Eligibility Budget Just In Time Post Award Activities 1 So What s
Ins and Outs of SBIR Grants and Contracts A Grants Manager Perspective Andre D. Walker Outline Differences Between a Grant and Contract Eligibility Budget Just In Time Post Award Activities 1 So What s
CITI REGISTRATION Step-by-step tutorial
 CITI REGISTRATION Step-by-step tutorial About CITI The Collaborative Institutional Training Initiative (CITI Program) at the University of Miami is a leading provider of research education content. Their
CITI REGISTRATION Step-by-step tutorial About CITI The Collaborative Institutional Training Initiative (CITI Program) at the University of Miami is a leading provider of research education content. Their
Dept/ College. Dept/ College. Dept/ College
 Office of Research Administration Matrix Revision date -2.10.17 Submitting proposals, executing awards, conducting research, and administrating sponsored projects involves many different people and units
Office of Research Administration Matrix Revision date -2.10.17 Submitting proposals, executing awards, conducting research, and administrating sponsored projects involves many different people and units
Save the Date: January 25, 2018 New Faculty Research Orientation
 Save the Date: January 25, 2018 New Faculty Research Orientation January 25, 2018 at 3:00PM Milo Bail Student Center The Office of Research and Creative Activity (ORCA) invites you to save the date for
Save the Date: January 25, 2018 New Faculty Research Orientation January 25, 2018 at 3:00PM Milo Bail Student Center The Office of Research and Creative Activity (ORCA) invites you to save the date for
Privacy Board Standard Operating Procedures
 Privacy Board Standard Operating Procedures Page 1 of 12 I. Background The Health Insurance Portability and Accountability Act ( HIPAA ) generally requires specific compliance reviews and documentation
Privacy Board Standard Operating Procedures Page 1 of 12 I. Background The Health Insurance Portability and Accountability Act ( HIPAA ) generally requires specific compliance reviews and documentation
Page 1 of 10 1/31/2008
 Page 1 of 10 1/31/2008 Step by Step Tips to Avoid Errors and Warnings o Full instructions can be found at: http://grants1.nih.gov/grants/funding/424/index.htm. o This document is intended to assist you
Page 1 of 10 1/31/2008 Step by Step Tips to Avoid Errors and Warnings o Full instructions can be found at: http://grants1.nih.gov/grants/funding/424/index.htm. o This document is intended to assist you
Research Financial Operations
 ASRSP/Cost Studies and Post Award Update Michael Daniels ASRSP Semi-annual Networking Event June 2016 Research Financial Operations Research Financial Operations Accounting Services for Research and Sponsored
ASRSP/Cost Studies and Post Award Update Michael Daniels ASRSP Semi-annual Networking Event June 2016 Research Financial Operations Research Financial Operations Accounting Services for Research and Sponsored
ROUTING SHEET. Principal Investigator: Department/Room No. Co-Principal Investigator: Title of the Project: SPONSOR INFORMATION: Sponsor due date:
 ROUTING SHEET IMPORTANT: The grant proposal or contract documents and a completed and signed routing sheet must be submitted to the Pre-Award office at least 5 (FIVE) working days prior to the date when
ROUTING SHEET IMPORTANT: The grant proposal or contract documents and a completed and signed routing sheet must be submitted to the Pre-Award office at least 5 (FIVE) working days prior to the date when
Export Controls. Internal Audit Report. Report No. SC June James Dougherty Principal Auditor
 Internal Audit Report Export Controls Report No. SC-13-15 June 2013 James Dougherty Principal Auditor Approved Barry Long, Director Internal Audit & Advisory Services Table of Contents I. EXECUTIVE SUMMARY...
Internal Audit Report Export Controls Report No. SC-13-15 June 2013 James Dougherty Principal Auditor Approved Barry Long, Director Internal Audit & Advisory Services Table of Contents I. EXECUTIVE SUMMARY...
Office of Sponsored Programs Workshop NIH Conflict of Interest Workshop 2
 Office of Sponsored Programs Workshop NIH Conflict of Interest Workshop 2 August 9, 2012 August 13, 2012-1 OSP Workshop Agenda Where we left off in Workshop 1 Who is an Investigator Conflicts of Interest
Office of Sponsored Programs Workshop NIH Conflict of Interest Workshop 2 August 9, 2012 August 13, 2012-1 OSP Workshop Agenda Where we left off in Workshop 1 Who is an Investigator Conflicts of Interest
Office of Commercialization and Innovation: Contracts & Industry Agreements (CIA)
 Office of Commercialization and Innovation: Contracts & Industry Agreements (CIA) Introduction to CIA and Contract Processing College of Engineering November 13, 2015 1 Overview of Today s Session 1. Introduction
Office of Commercialization and Innovation: Contracts & Industry Agreements (CIA) Introduction to CIA and Contract Processing College of Engineering November 13, 2015 1 Overview of Today s Session 1. Introduction
West Virginia Clinical and Translational Science Institute Open Competition RFA
 West Virginia Clinical and Translational Science Institute Open Competition RFA Part 1. Overview Information The goal of this Request for Applications (RFA) is to support clinical and translational pilot
West Virginia Clinical and Translational Science Institute Open Competition RFA Part 1. Overview Information The goal of this Request for Applications (RFA) is to support clinical and translational pilot
EXPORT CONTROL MANAGEMENT PROGRAM
 EXPORT CONTROL MANAGEMENT PROGRAM Revised August 2012 The University of Iowa Export Control Management Program Table of Contents Introduction... 2 University of Iowa Policy on Export Control Management...
EXPORT CONTROL MANAGEMENT PROGRAM Revised August 2012 The University of Iowa Export Control Management Program Table of Contents Introduction... 2 University of Iowa Policy on Export Control Management...
APPLICATION FOR RESEARCH GRANT TITLE OF RESEARCH. Amount requested Dates of study: Begin End. Name. Title. Department. Name of Department Chair
 THE HITCHCOCK FOUNDATION 1 Medical Center Drive Lebanon, NH 03756-0001 Phone (603) 653-0481 Fax (603) 676-4331 Jennifer.k.reining@hitchcock.org APPLICATION FOR RESEARCH GRANT TITLE OF RESEARCH Amount requested
THE HITCHCOCK FOUNDATION 1 Medical Center Drive Lebanon, NH 03756-0001 Phone (603) 653-0481 Fax (603) 676-4331 Jennifer.k.reining@hitchcock.org APPLICATION FOR RESEARCH GRANT TITLE OF RESEARCH Amount requested
Safety Approval for Research Proposals
 Approval for Research Proposals Policy Health and safety issues must be considered in the planning and design of research work. Adding on safety measures later in the project rarely ever works, can be
Approval for Research Proposals Policy Health and safety issues must be considered in the planning and design of research work. Adding on safety measures later in the project rarely ever works, can be
10 STEPS TO IRB APPROVAL
 10 STEPS TO IRB APPROVAL STEP 1: OBTAIN CITI TRAINING CERTIFICATE ALL STUDY PERSONNEL MUST TAKE AN ONLINE HUMAN SUBJECTS TRAINING EDUCATION PROGRAM. THE CERTIFICATES OF COMPLETION ARE NEEDED FOR ALL STUDY
10 STEPS TO IRB APPROVAL STEP 1: OBTAIN CITI TRAINING CERTIFICATE ALL STUDY PERSONNEL MUST TAKE AN ONLINE HUMAN SUBJECTS TRAINING EDUCATION PROGRAM. THE CERTIFICATES OF COMPLETION ARE NEEDED FOR ALL STUDY
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research
 Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director Craig O Neill, Office of Sponsored Programs, Manager February
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director Craig O Neill, Office of Sponsored Programs, Manager February
Outgoing Subagreements: Subawards and Subcontracts
 Effective Date: March 1, 2014 Version: 1 Page: 1 of 6 Overview Sponsored programs may include partnerships with other organizations or institutions whereby a portion of the project is conducted by investigators
Effective Date: March 1, 2014 Version: 1 Page: 1 of 6 Overview Sponsored programs may include partnerships with other organizations or institutions whereby a portion of the project is conducted by investigators
Get Funded. We are here to help! Funding Support: Michelle Williams, AVPR (860)
 Research@UConn The Office of the Vice President for Research (OVPR) is responsible for overseeing the $260 million annual research enterprise across all UConn campuses including UConn Health and manages
Research@UConn The Office of the Vice President for Research (OVPR) is responsible for overseeing the $260 million annual research enterprise across all UConn campuses including UConn Health and manages
Cayuse SP: DETAILED GUIDANCE FOR 2018 URCF PROPOSALS
 Before you begin Please observe the following guidance. All applicants and any named personnel must be registered as users in Cayuse before starting a proposal in Cayuse SP. This will enable users to access
Before you begin Please observe the following guidance. All applicants and any named personnel must be registered as users in Cayuse before starting a proposal in Cayuse SP. This will enable users to access
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research
 Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director February 24, 2017 1 Agenda A Walkthrough of MSU s Proposal
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director February 24, 2017 1 Agenda A Walkthrough of MSU s Proposal
Financial Research Compliance. April 2013
 Financial Research Compliance April 2013 Overview I. What is a Sponsored Award? II. Sponsored Awards at WFU III. Compliance IV. Hot Topics V. Audits VI. WFU Updates VII. Questions VIII. Contacts What is
Financial Research Compliance April 2013 Overview I. What is a Sponsored Award? II. Sponsored Awards at WFU III. Compliance IV. Hot Topics V. Audits VI. WFU Updates VII. Questions VIII. Contacts What is
ANTHROPOLOGY GRADUATE STUDENT WORKSHOP
 ANTHROPOLOGY GRADUATE STUDENT WORKSHOP Weinberg Research Administration February 1 2016 http://www.weinberg.northwestern.edu/research/administration/index.html December, 2013 Research Administration in
ANTHROPOLOGY GRADUATE STUDENT WORKSHOP Weinberg Research Administration February 1 2016 http://www.weinberg.northwestern.edu/research/administration/index.html December, 2013 Research Administration in
MDF Request for Applications (RFA) AWARD POLICY
 MDF Request for Applications (RFA) AWARD POLICY The Myotonic Dystrophy Foundation (MDF) is the world s largest patient organization focused solely on myotonic dystrophy. Our mission, Care and a Cure, is
MDF Request for Applications (RFA) AWARD POLICY The Myotonic Dystrophy Foundation (MDF) is the world s largest patient organization focused solely on myotonic dystrophy. Our mission, Care and a Cure, is
Presented by NC TraCS Institute UNC Office of Clinical Trials UNC Network of Clinical Research Professionals
 ORIENTATION FOR NEW CLINICAL RESEARCH COORDINATORS Presented by NC TraCS Institute UNC Office of Clinical Trials UNC Network of Clinical Research Professionals Overall Agenda for Orientation Module 1:
ORIENTATION FOR NEW CLINICAL RESEARCH COORDINATORS Presented by NC TraCS Institute UNC Office of Clinical Trials UNC Network of Clinical Research Professionals Overall Agenda for Orientation Module 1:
Expectations on the Occupational Health and Safety Programme (OHSP)
 Expectations on the Occupational Health and Safety Programme (OHSP) Bryan Howard, MRCVS, BVMS, M.Sc., M.Ed. Officer, Council on Accreditation AAALAC International Exercise: Talk with those near you and
Expectations on the Occupational Health and Safety Programme (OHSP) Bryan Howard, MRCVS, BVMS, M.Sc., M.Ed. Officer, Council on Accreditation AAALAC International Exercise: Talk with those near you and
