Marcia Smith Associate Vice Chancellor for Research
|
|
|
- Collin Curtis
- 5 years ago
- Views:
Transcription
1 Research Administrators Forum February 9, 2012 Marcia Smith Associate Vice Chancellor for Research
2 Agenda Welcome and Announcements Marcia Smith Data Management Tool Sharon Farb, Associate University Librarian, and Todd Grappone, Associate University Librarian for Digital Initiatives and IT OHRPP/Office of Radiation Safety Committees Kathy Wadsworth OCGA Initiatives Patti Manheim Streamlining Proposal and Award Processes Phase I - Report on Award Intake Pilot Project Streamlining Proposal and Award Processes Phase II - Proposal Deadline Statistics and Notices, Minimum Proposal Submission Requirements and Phasing In Proposal Intake NIH Salary Cap Guidance for Proposals and Awards EFM Initiatives Tracey Robertson NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout Department Threshold for Recertification RPC Update Ann Pollack Policy 900 PI Eligibility
3 Upcoming ORA Training Opportunities Proposal Preparation & Submission February 22, :00 am 3:30 pm Subawards in S2S Grants February 23, :00 am - Noon Post Award Administration March 28, :00 am 3:30 pm Effort Reporting April 2, :00 am 3:30 pm Rapid Close-Out Tool April 3, :00 am - Noon
4 Please fill out the survey forms
5 DMPTool for Data Management Plans Todd Grappone and Sharon E. Farb Presented to the Research Administrators Forum (RAF) February 9, 2012
6 NSF Awards to UCLA in FY $84,364, % of awarded dollars to UCLA come from NSF
7 Federal Dollars for Science and Engineering UCLA was ranked 5 th in 2007 (most recent data available from NSF) UCLA is consistently ranked in the top 10
8 NSF Requirement for Data Management Plan (DMP) Proposals must include a supplementary document of no more than two pages labeled Data Management Plan. This supplement should describe how the proposal will conform to NSF policy on the dissemination and sharing of research results.
9 Reasons for the DMPTool NSF requirements for data management plans beginning Jan 2011 For instance, University of California researchers received over $600 million from NSF in FY 2010/11 Other agencies following suit: NEH, IMLS NIH has data sharing requirements February 9, 2012
10 UCLA Project participants Todd Grappone Judy Consales Sharon Farb Lisa Federer Courtney Hoffner Tony Aponte Anita Colby Claudia Horning Jen Weintraub Stephen Davison Gary Thompson Darrow Cole Dawn Setzer February 9, 2012
11 Data Management this concept refers to the activities in the research lifecycle that involve some aspect of planning, collecting, processing, editing, preparing, documenting, verifying, analyzing, preserving, discovering and repurposing data; a Data Management Plan should articulate how these data activities will be conducted in a research project; The WHAT of e-research data activities
12 Data Stewardship this concept refers to the individuals, parties or institutions taking responsibility for data management activities across the research lifecycle; a Data Management Plan should identify the data stewards associated with a research project; The WHO involved in e-research data activities
13 DMPTool for Data Management Plans Helps researchers meet requirements of NSF and other U.S. funding agencies. Guides researchers through the process of creating a data management plan. Is available to everyone. Provides additional help for researchers at DMPTool partner institutions like UCLA. data@library.ucla.edu February 9, 2012
14 NSF Award & Administration Guide NSF Dissemination and Sharing Policy Investigators are expected to share with other researchers, at no more than incremental cost and within a reasonable time, the primary data, samples, physical collections and other supporting materials created or gathered in the course of work under NSF grants. Grantees are expected to encourage and facilitate such sharing.
15 Goals of the DMPTool, I To provide researchers a simple way to create a Data Management Plan by giving them information from the funding agency: Questions asked by the agency Any additional explanation or context provided by the agency Links to the agency website for policies, help, guidance data@library.ucla.edu February 9, 2012
16 Goals of the DMPTool, II To provide researchers with additional information from their local institution: Resources and services to help them manage data Help text for specific questions Suggested answers to questions that they can simply cutand-paste News and events related to data management on their campus February 9, 2012
17
18
19
20
21
22
23
24
25 Add local information Help text, Links to resources and services, Suggested answers, Contact information Information can be added at various levels for researchers at UCLA: All data management plans All data management from a particular funding agency, e.g.. NSF Biological Sciences Directorate A particular question within a data management plan data@library.ucla.edu February 9, 2012
26 Questions? Contact us at to participate Important links: Funder Templates: DMPTool Blog: February 9, 2012
27 (easy-eye-dee) Create a persistent identifier: DOI or ARK Add object location Add metadata Update object location Update object metadata
28 The New Group at ORA Kathy Wadsworth ORSC and OHRPP Policy & Education
29 Need for Increased Oversight
30 What is the ORSC? Provides administrative support for the Radiation Safety SaeyCommittee ee( (RSC) and 4 subcommittees: Medical Radiation Safety Committee (MRSC) Radioactive Drug Research Committee (RDRC) * New subcommittees: Academic Radiation Safety Committee (ARSC) Clinical Operations Radiation Safety Committee (CORSC)
31
32
33 Patti Manheim, Director Patti Manheim, Director February 09, 2012
34 STEAMLINING PROPOSAL AND AWARD PROCESSES PHASE I Award Intake and Set-Up Pilot Initiated: OCGA launched a joint initiative focused on improving processing timelines for unilateral awards and receipt and tracking of complex awards Awards Processed to Date: 458 awards Average Turnaround Time: 3.6 days from Award receipt to account set up
35 STEAMLINING PROPOSAL AND AWARD PROCESSES Award Set Up Time Has Improved by 80%
36 STEAMLINING PROPOSAL AND AWARD PROCESSES Benefits: Quicker Access to Funds Timely and Consistent Communication at Key Process Points: Award Received Award Set Up Complete Key Data to Understand: Where an Award is in the Process What is Holding Up Activation Reduction of retroactive transactions due to late account set-up
37 STEAMLINING PROPOSAL AND AWARD PROCESSES PHASE II Proposal Intake and Processing 60% of the proposals received by OCGA are considered incomplete Incomplete proposals risk the following: Delayed award set-up Insufficient time for a meaningful OCGA review because we are waiting for at least the minimum documents Full compliance with sponsor regulations is not ensured if we do not have the sponsor regulations Insufficient time to correct errors/validations that arise from sponsor systems
38 STEAMLINING PROPOSAL AND AWARD PROCESSES OCGA will pilot the following Proposal Intake Process with select departments: Specific staff will become the central contact for receipt of all proposals OCGA will conduct an initial review to confirm minimum documents are included: d Do not meet minimum requirements: the proposal cannot be reviewed and missing documents will be requested Does meet minimum requirements: the compliant the proposal package will be assigned to OCGA team for review Standard communication at key process points Once PATS is deployed, we will be able to provide real time data for proposals on the ORA portal
39 STEAMLINING PROPOSAL AND AWARD PROCESSES
40 STEAMLINING PROPOSAL AND AWARD PROCESSES Proposal Intake Pilot First Steps: Identify pilot departments Meet with departments to discuss process and answer questions Confirm departments t understanding di and agreement
41 STEAMLINING PROPOSAL AND AWARD PROCESSES Proposal Intake Pilot Next Steps: Pilot begins Measure intake and processing timelines Share feedback from pilot participants Phase in all departments t
42 DANGER IN LATE PROPOSAL SUBMISSION Complete proposal packages are due into OCGA 5 Business Days prior to Sponsor Deadline Over 75% of our monthly proposal volume is received within 0-3 days of the sponsor deadline
43 DANGER IN LATE PROPOSAL SUBMISSION Risks - If the proposal package is not received within this timeframe, we risk the following: Sponsor rejection of incomplete proposals Invalidation and system errors Underfunding due to budget errors Compliance issues that would cause UCLA to reject the award Delays in processing awards
44 DANGER IN LATE PROPOSAL SUBMISSION NIH Warns Potential Dangers when submitting an application near the deadline.
45 DANGER IN LATE PROPOSAL SUBMISSION Single grant analyst received 25 graduate student grant applications within 24 hours of the sponsor deadline No time to review anything even budgets F&A not properly requested Non scientific received early and reviewed; research plan uploaded five minutes prior to deadline No time to open and review attachment After submission and deadline, PI noticed a font color had been changed No chance to submit a change
46 DANGER IN LATE PROPOSAL SUBMISSION Electronic application submitted 5 minutes prior to sponsor deadline Not time to review System errors and page limitations No opportunity to resubmit
47 DANGER IN LATE PROPOSAL SUBMISSION HELP US HELP YOU: Complete proposal packages are due into OCGA 5 Business Days prior to the sponsor deadline date At any other time prior to the 5 day deadline, you can submit a proposal package that includes the minimum required documents Advantages: Expedited award processing Sufficient time for review and correction of system errors Project costs are correctly calculated General happiness
48 NIH SALARY CAP Effective 12/23/2011 Decreased Salary Cap - Executive Level II - $179, Link to Notice: files/not-od html All applications to all DHHS Operating Divisions not just NIH, AHRQ and SAMSHA - should not exceed this salary cap What does this mean for our current awards?
49 NIH SALARY CAP Type of FY 2012 Award Received Executive Level I $199,700 Executive Level II $179,700 What Happens to My Awarded Budget? Competitive (New and Renewal) X No decrease in award will be made for first year as Executive Level I with Initial Issue Date on/before salary applies. 12/22/2011 Future years will be decreased to adjust to the Executive Level II salary. Non Competing with Initial Issue Date on/before 12/22/2011 Competitive (New and Renewal) with Initial Issue Date on/after 12/23/2011 Non Competing with Initial Issue Date on/after 12/23/2011 X No decrease in award will be made for the current year and Executive Level I salary applies. Future years will not be decreased to adjust to the Executive Level II, but Executive Level II salary will apply. Funds may be re budgeted elsewhere. X Award will be decreased to adjust to the Executive Level II, and the Executive Level II salary applies. Future years will be decreased to adjust to the Executive Level II. X The award made with federal FY2012 funds will not be decreased to adjust to the Executive Level II, but the Executive Level II salary applies. Future years will also not be adjusted down. Funds for all years may be re budgeted elsewhere. FY2011 and prior Awards X Awards made with federal FY2011 funds, including carry forward funds from FY2011 and prior, Executive Level I salary applies.
50 NCRR Publication Acknowledgement The transfer of NIH grants from the National Center for Research Resources (NCRR) to other NIH funding components has led to several questions about the acknowledgement language to be used in publications, press releases, etc. NIH has requested we use the following language: This project was supported by the National Center for Research Resources and the [new funding component] of the National Institutes of Health through Grant Number XXXXX.
51 EFM Updates NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout - Department Threshold for Recertification 1
52 EFM Updates NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout - Department Threshold for Recertification 2
53 Salary Cap Guidance The use of current FY2011 funds and carry-forward funds from FY 2011 and prior fiscal years can be used to pay salary at the Executive Level 1 rate of $199,700. EFM is in the process of developing a report that will help us monitor funds that will continue to use the Executive Level I salary cap. EFM will review anyone paid at the Executive Level 1 rate of $199,700 to ensure that it complies with the terms of the award. 3
54 EFM Updates NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout - Department Threshold for Recertification 4
55 SFN - Benefits Use of Single Fund Number started February 1, 2012: Long-standing practice called for certain awards such as Program Project Grants, Training Grants, and Cooperative Agreements to be assigned a new fund number for each year of the award. The Single Fund number transitions to one fund number per award for all awards. Reduces administrative burden and costs campus wide Elimination of an estimated 1,000 fund set-up and close-outs each year Faster activation of continuation awards for PI s Decrease in the number of cost transfers Increase in on-time submission of reports and invoices Decrease in the number of revised financial reports. Decrease in the number of required pre-award spending accounts (RAS) Decrease in recycled fund numbers Decrease in required changes in recharge IDs for PIs 5
56 SFN - Benefits On January 30th a memo was sent to ORA News announcing the implementation of single fund number and the many benefits of a transition to single fund number. We have received some positive feedback: Thank you for championing this change! This is great news. I also really appreciate the detailed description of the benefits, and I know my faculty will as well. You have made many PIs and Research Administrators very happy today 6
57 SFN - FAQ s The process for implementing Single Fund Number has been discussed extensively with, and developed by, representatives of RAPID work groups, campus committees, ORA staff, and members of the RAPID Steering and Faculty Advisory Committees. Throughout the process we tracked and developed FAQ documentation to aid in the transition to Single Fund Number. The FAQ s were sent out on January 30 th in the ORA News announcement and can also be found on the New EFM Website: 7
58 SFN - FAQ s Page # 8
59 SFN - System Notifications Language on the 90, 30, and 0 day notices has been updated to represent the budget period and not the project period. These notices are more generic so please pay attention to your budget versus your project period end date. Notification Attention and Subject 90 Day Notification To: PI Cc: Department Administrator Subject: IMPORTANT NOTICE: Sponsored Award Budget Period Expires in 90 Days 30 Day Notification To: Department Administrator Cc: PI Subject: IMPORTANT NOTICE: Sponsored Award Budget Period Expires in 30 Days Fund Expiration Notification Subject: IMPORTANT NOTICE: Sponsored Award Budget Period Has Expired 9
60 SFN - System Notifications Please complete all necessary actions below: ENSURE ALL DELIVERABLES HAVE BEEN COMPLETED AND SUBMITTED TO SPONSOR (including): Progress Reports, Invention Statements, Technical Reports FUND IS TO REMAIN OPEN: Please work with your OCGA/OCT/OIP and EFM contacts, as needed, on the following: Non-Competing Continuations Amendment Request (Renewal, Additional Funding, etc.) No Cost Time Extension Request Carry Forward Request Collect Final Subawardee Invoice for the budget period (all final subawardee invoices are due to UCLA 45 days after the budget end date) FUND IS READY TO CLOSE: Complete the RAPID Smart Closeout Tool and submit to EFM by the deadline. The tool can be downloaded here: 10
61 SFN Process Process for Interim Financial Reports EFM completes the financial report based on the General Ledger expenses EFM reviews the unallowables and will send the Interim Report and a list of the unallowables to the department fund manager Dept. fund manager has 5 days to review and approve the interim i financial i report and respond to EFM If dept. does not respond within 5 days EFM will submit the interim report (excluding the unallowables) to the sponsor Process for Restricted Carry-Forwards: EFM completes the financial report, indicating carry-forward amount to be requested EFM will de-appropriate the funds from the current year and move the funds to a carry-forward holding account (400005) linked to the current fund Dept. continues to work with OCGA to request carry-forward Once approved, funds will be re-appropriated and removed from carry-forward holding account 11
62 SFN Exception Request Form Exceptions to this new process will be granted on a very limited basis Requests for exceptions can be submitted for review using the Exception Request Form (also sent out on January 30 th in the ORA News and can be found on the EFM website) Form should be completed and submitted to the intake team - oraawards@research.ucla.edu The form will be reviewed by ORA Leadership 12
63 SFN Exception Request Form 13
64 EFM Updates NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout - Department Threshold for Recertification 14
65 ARRA Updates Memo will be sent to the ARRA Listserv: OMB will limiting no-cost extensions for funds beyond September 30, 2013 Other agencies have released notices indicating that they will not allow nocost extensions for ARRA funds beyond September 30, Prior written approval to extend beyond the September 30, 2013 date will only be considered if one or more of the following circumstances exist: The project is long-term by design, and acceleration would compromise core programmatic goals. The project must undergo a complex environmental review that cannot be completed within this time frame. Contractual commitments by the grantee with vendors or subrecipients prevent adjusting the timeline for spending. Other special circumstances may exist 15
66 ARRA Updates Recent letters from NSF have requested that PIs with ARRA Awards that (1) extend beyond September 30, 2013 or (2) are eligible for no cost-extensions that will extend the award beyond September 30, 2013 submit a written request for the extension to their Program Officer The requests that we have seen from NSF have a due date of March 2 nd. Please review all your ARRA awards and contact your Program Officer as soon as possible if you have awards that: 1) Extend beyond Sept. 30, ) End before Sept. 30, 2013 but you will anticipate the need for a no-cost extension 16
67 ARRA Updates EFM will be following-up with reminders to specific PIs who have ARRA awards which: Have end dates that extend beyond September 30, 2013 or Have a balance greater than 100k 17
68 EFM Updates NIH Salary Cap Guidance for Post Award Management Single Fund Number Initiative ARRA Funds and Spending Timelines Fund Closeout - Department Threshold for Recertification 18
69 Department Threshold PI Threshold remains $500 As of February 1 st we have implemented a department threshold for recertification of closeout packets of $100 What does this mean? Department submits their closeout packet to EFM. EFM reviews the closeout packet and the final number differs from the department by ><$100. EFM will not be required to receive re-approval from the department and can submit the final invoice and/or financial report to the sponsor. EFM will the final invoice and/or financial report to the department. 19
70 Ann Pollack Assistant Vice Chancellor Research February 9, 2012
71 UCLA Policy 900: Principal Investigator Eligibility Sets forth the eligibility requirements, duties and responsibilities of a UCLA Principal Investigator Describes the processes for requesting and approving exceptions to the eligibility requirements
72 UCLA Policy 900: Principal Investigator Eligibility Updates effective January 17, 2012 Changes made in response to feedback from the Graduate Division and departments Primary changes affect postdoctoral scholars y g p and other trainees who may not normally serve as a PI, Co-PI or Multiple PI
73 UCLA Policy 900: Principal Investigator Eligibility Policy amended to reflect UCLA s recognition of the fact that proposal preparation is an important aspect of training Policy now indicates that postdoctoral scholars and other trainees may apply for research training and mentored training grants that help enhance their professional skills and prepare them for research independence d
74 UCLA Policy 900: Principal Investigator Eligibility Other changes made to provide clarification and Other changes made to provide clarification and to emphasize the fact that academic units must provide space and access to facilities, and take responsibility for effectively managing projects when they support PI exception requests
75 UCLA Policy 900: Principal Investigator Eligibility Requests for exceptions: Reviewed and approved by campus officials with authority to grant exceptions (see UCLA Delegation of Authority ) Individual schools may make local decisions about review of PI exception requests
76 Questions or Comments Please send any yquestions or comments to: 20
Please fill out the survey forms 2/9/2012. Upcoming ORA Training Opportunities. Agenda
 Agenda Welcome and Announcements Marcia Smith Research Administrators Forum February 9, 2012 Marcia Smith Associate Vice Chancellor for Research Data Management Tool Sharon Farb, Associate University Librarian,
Agenda Welcome and Announcements Marcia Smith Research Administrators Forum February 9, 2012 Marcia Smith Associate Vice Chancellor for Research Data Management Tool Sharon Farb, Associate University Librarian,
Research Administrators Forum May 8th, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum May 8th, 2014 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Office of Research Administration OHRPP Retention of Research
Research Administrators Forum May 8th, 2014 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Office of Research Administration OHRPP Retention of Research
Research Administration Forum March 9, Marcia Smith Associate Vice Chancellor for Research
 Research Administration Forum March 9, 2017 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements - Marcia Smith Immigration Update and Q & A Sam Nahidi, Assistant Director,
Research Administration Forum March 9, 2017 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements - Marcia Smith Immigration Update and Q & A Sam Nahidi, Assistant Director,
Sponsored by Office of Research Administration. Marcia Smith Associate Vice Chancellor Research Administration. July 9, 2009
 Research Administrators Forum Sponsored by Office of Research Administration Marcia Smith Associate Vice Chancellor Research Administration July 9, 2009 Agenda 2 Updates from the Animal Research Committee
Research Administrators Forum Sponsored by Office of Research Administration Marcia Smith Associate Vice Chancellor Research Administration July 9, 2009 Agenda 2 Updates from the Animal Research Committee
Research Administrators Forum August 14th, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum August 14th, 2014 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Associate Vice Chancellor, Office of Research Administration
Research Administrators Forum August 14th, 2014 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Associate Vice Chancellor, Office of Research Administration
Sponsored by Office of Research Administration
 Research Administrators Forum Sponsored by Office of Research Administration Marcia Smith Associate Vice Chancellor Research Administration January 14, 2010 Meeting Agenda Meeting Agenda Welcome and Announcements
Research Administrators Forum Sponsored by Office of Research Administration Marcia Smith Associate Vice Chancellor Research Administration January 14, 2010 Meeting Agenda Meeting Agenda Welcome and Announcements
Updates on American Recovery and Reinvestment Act (ARRA) Funding. Virginia Anders, Acting Director, OCGA Evelyn Balabis, Director, EFM
 Updates on American Recovery and Reinvestment Act (ARRA) Funding Virginia Anders, Acting Director, OCGA Evelyn Balabis, Director, EFM 15 OCGA ARRA Help Desk 10:00 a.m. 5:00 p.m., Monday Friday 310-794-0548
Updates on American Recovery and Reinvestment Act (ARRA) Funding Virginia Anders, Acting Director, OCGA Evelyn Balabis, Director, EFM 15 OCGA ARRA Help Desk 10:00 a.m. 5:00 p.m., Monday Friday 310-794-0548
Patti Manheim, Director, OCGA
 Research Administrators Forum Patti Manheim, Director, OCGA Agenda Welcome - Patti Manheim OCGA Updates Patti Manheim * Consortium/contractual arrangements versus purchase of services * Master Calendar
Research Administrators Forum Patti Manheim, Director, OCGA Agenda Welcome - Patti Manheim OCGA Updates Patti Manheim * Consortium/contractual arrangements versus purchase of services * Master Calendar
Patti Manheim, Director April 11, 2013
 Patti Manheim, Director April 11, 2013 Today s Topics NIH Research Performance Project Report (RPPR) Update Patti Manheim NIH Public Access Policy Reporting Requirements Patti Manheim and Lisa Federer,
Patti Manheim, Director April 11, 2013 Today s Topics NIH Research Performance Project Report (RPPR) Update Patti Manheim NIH Public Access Policy Reporting Requirements Patti Manheim and Lisa Federer,
Research Administrators Forum September 13, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum September 13, 2012 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith OCGA Initiatives Patti Manheim, Kim Duiker Proposal Intake
Research Administrators Forum September 13, 2012 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith OCGA Initiatives Patti Manheim, Kim Duiker Proposal Intake
Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards
 Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards Preparing campus for what is changing and what is not What is Grants Reform and when is it effective? What
Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards Preparing campus for what is changing and what is not What is Grants Reform and when is it effective? What
FEDERAL FUND CLOSEOUT. RAF November 12, 2015 Yoon Lee
 FEDERAL FUND CLOSEOUT RAF November 12, 2015 Yoon Lee Agenda Key Steps in the Procedure September and October FFR Submission Frequently Asked Questions December Deadlines 2 Federal Fund Closeout Key Steps
FEDERAL FUND CLOSEOUT RAF November 12, 2015 Yoon Lee Agenda Key Steps in the Procedure September and October FFR Submission Frequently Asked Questions December Deadlines 2 Federal Fund Closeout Key Steps
Research Administrators Forum August 8th, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum August 8th, 2013 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Office of Research Administration Conflicts of Interest
Research Administrators Forum August 8th, 2013 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith, Office of Research Administration Conflicts of Interest
Sponsored Programs Roles & Responsibilities
 The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
Research Administrators Forum October 10th, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum October 10th, 2013 Marcia Smith Associate Vice Chancellor for Research Agenda Meeting Agenda Welcome and Announcements Marcia Smith, Office of Research Administration Contract
Research Administrators Forum October 10th, 2013 Marcia Smith Associate Vice Chancellor for Research Agenda Meeting Agenda Welcome and Announcements Marcia Smith, Office of Research Administration Contract
Sponsored Programs Roles & Responsibilities
 The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
Patti Manheim - Director February 13, 2014
 Patti Manheim - Director February 13, 2014 Today s Topics NIH Fiscal Operations for FY 2014 Updates from the January 2014 FDP Meeting Other OCGA Updates NIH Fiscal Operations for FY2014 NIH Budget $30.15
Patti Manheim - Director February 13, 2014 Today s Topics NIH Fiscal Operations for FY 2014 Updates from the January 2014 FDP Meeting Other OCGA Updates NIH Fiscal Operations for FY2014 NIH Budget $30.15
Agenda. NIH Update. Other Updates. Proposal & Progress Report Statistics Research Administration Training Topic:
 Agenda NIH Update Salary Cap Guidance NOT OD 17 037: NIH Implementation of the Interim RPPR While a Renewal Application is Under Consideration Prior Approval for No Cost Extensions via the era Commons
Agenda NIH Update Salary Cap Guidance NOT OD 17 037: NIH Implementation of the Interim RPPR While a Renewal Application is Under Consideration Prior Approval for No Cost Extensions via the era Commons
OSR Monthly Meeting. August 16, 2018 (Chicago) August 21, 2018 (Evanston)
 OSR Monthly Meeting August 16, 2018 (Chicago) August 21, 2018 (Evanston) Agenda OSR Announcements/Staffing Updates ESPR Replacement Project Update Year-end Procedures NSF Updates NIH Biosketch Reminder
OSR Monthly Meeting August 16, 2018 (Chicago) August 21, 2018 (Evanston) Agenda OSR Announcements/Staffing Updates ESPR Replacement Project Update Year-end Procedures NSF Updates NIH Biosketch Reminder
Sponsored Programs New Developments and Important Reminders
 Sponsored Programs New Developments and Important Reminders Rebecca Trahan Office of Sponsored Programs March 7, 2012 1 Training Session Overview National Institutes of Health (NIH) Update NSF Proposal
Sponsored Programs New Developments and Important Reminders Rebecca Trahan Office of Sponsored Programs March 7, 2012 1 Training Session Overview National Institutes of Health (NIH) Update NSF Proposal
Subrecipient Monitoring Procedures
 Subrecipient Monitoring Procedures This procedure describes the proper management of subrecipient activity under Purdue sponsored program awards. Definitions Award: An award is a binding agreement between
Subrecipient Monitoring Procedures This procedure describes the proper management of subrecipient activity under Purdue sponsored program awards. Definitions Award: An award is a binding agreement between
2017 State of Connecticut Regenerative Medicine Research Fund (RMRF) General RFP
 2017 State of Connecticut Regenerative Medicine Research Fund (RMRF) General RFP (Seed, Established Investigator, Group) Full RFP, Forms and additional FAQs: http://ctinnovations.com/rmrf Deadline Friday,
2017 State of Connecticut Regenerative Medicine Research Fund (RMRF) General RFP (Seed, Established Investigator, Group) Full RFP, Forms and additional FAQs: http://ctinnovations.com/rmrf Deadline Friday,
FEDERAL FUND CLOSEOUT. RAF August 13, 2015 Yoon Lee
 FEDERAL FUND CLOSEOUT RAF August 13, 2015 Yoon Lee Agenda Communication update Applicability and population Next steps Resources 2 Federal Fund Closeout Procedure: Communication Update April 2015 Presented
FEDERAL FUND CLOSEOUT RAF August 13, 2015 Yoon Lee Agenda Communication update Applicability and population Next steps Resources 2 Federal Fund Closeout Procedure: Communication Update April 2015 Presented
University of San Francisco Office of Contracts and Grants Subaward Policy and Procedures
 Summary 1. Subaward Definitions A. Subaward B. Subrecipient University of San Francisco Office of Contracts and Grants Subaward Policy and Procedures C. Office of Contracts and Grants (OCG) 2. Distinguishing
Summary 1. Subaward Definitions A. Subaward B. Subrecipient University of San Francisco Office of Contracts and Grants Subaward Policy and Procedures C. Office of Contracts and Grants (OCG) 2. Distinguishing
Research Administrators Forum November 8, Marcia Smith Associate Vice Chancellor for Research
 Research Administrators Forum November 8, 2012 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith RAPID Update PAMS Jessica Carney, Allison Philabaum Post
Research Administrators Forum November 8, 2012 Marcia Smith Associate Vice Chancellor for Research Agenda Welcome and Announcements Marcia Smith RAPID Update PAMS Jessica Carney, Allison Philabaum Post
Diane Dean, Director Kathy Hancock, Assistant Grants Compliance Officer Joel Snyderman, Assistant Grants Compliance Officer
 Division of Grants Compliance and Oversight Office of Policy for Extramural Research Administration, OER National Institutes of Health, DHHS NIH Regional Seminar San Diego, CA October 2015 Diane Dean,
Division of Grants Compliance and Oversight Office of Policy for Extramural Research Administration, OER National Institutes of Health, DHHS NIH Regional Seminar San Diego, CA October 2015 Diane Dean,
Today s Topics. OCGA Quick Updates Kim Duiker and Heather Winters Updates from NIH Regional Seminar Kim Duiker S2S Grants Updates Cindy Gilbert
 August 8, 2013 Today s Topics OCGA Quick Updates Kim Duiker and Heather Winters Updates from NIH Regional Seminar Kim Duiker S2S Grants Updates Cindy Gilbert Quick Updates OCGA Staffing Update Three retirements
August 8, 2013 Today s Topics OCGA Quick Updates Kim Duiker and Heather Winters Updates from NIH Regional Seminar Kim Duiker S2S Grants Updates Cindy Gilbert Quick Updates OCGA Staffing Update Three retirements
VCR Five Day Proposal Submission Policy Implementation Guidance RAC Forum March 16, 2016
 UC BERKELEY VCR Five Day Proposal Submission Policy Implementation Guidance RAC Forum March 16, 2016 Why did Berkeley need a new policy? Some PIs were using late proposals as SOP SUN faculty members noted
UC BERKELEY VCR Five Day Proposal Submission Policy Implementation Guidance RAC Forum March 16, 2016 Why did Berkeley need a new policy? Some PIs were using late proposals as SOP SUN faculty members noted
office of research administration newsletter
 july/august 2010 vol. 5 no. 1 office of research administration newsletter Yale has a clear obligation to comply with all regulations pertaining to the administration of federal grants, and we will spare
july/august 2010 vol. 5 no. 1 office of research administration newsletter Yale has a clear obligation to comply with all regulations pertaining to the administration of federal grants, and we will spare
Cost Sharing. Policy Statement and Purpose
 Cost Sharing Policy Type: Administrative Responsible Office: Office of Sponsored Programs (proposal and award), Office of Research and Innovation, and Grants and Contracts Accounting (fiscal and accounting),
Cost Sharing Policy Type: Administrative Responsible Office: Office of Sponsored Programs (proposal and award), Office of Research and Innovation, and Grants and Contracts Accounting (fiscal and accounting),
NSF 2 Month Handbook. Effective for Reviews Performed as of 07/01/17. NSF Account Management. Updated 07/24/17
 Updated 07/24/17 NSF 2 Month Handbook Effective for Reviews Performed as of 07/01/17 NSF Account Management Table of Contents Contents 2 Month Rule Policy... 2 NSF Definition... 2 Sponsor/University Guidance:...
Updated 07/24/17 NSF 2 Month Handbook Effective for Reviews Performed as of 07/01/17 NSF Account Management Table of Contents Contents 2 Month Rule Policy... 2 NSF Definition... 2 Sponsor/University Guidance:...
Uniform Guidance Sponsored Projects Services
 Arizona s First University. Uniform Guidance Sponsored Projects Services 520-626-6000 sponsor@email.arizona.edu Agenda What is Uniform Guidance (UG)? Effective dates Structure of the Uniform Guidance Significant
Arizona s First University. Uniform Guidance Sponsored Projects Services 520-626-6000 sponsor@email.arizona.edu Agenda What is Uniform Guidance (UG)? Effective dates Structure of the Uniform Guidance Significant
SJSU Research Foundation
 SJSU Research Foundation The Nuts & Bolts of Proposal Preparation and Award Management Sponsored Programs Workshop February 16, 2017 Agenda Discussion of Common Issues and Solutions Terms and Conditions
SJSU Research Foundation The Nuts & Bolts of Proposal Preparation and Award Management Sponsored Programs Workshop February 16, 2017 Agenda Discussion of Common Issues and Solutions Terms and Conditions
Accelerated Translational Incubator Pilot (ATIP) Program. Frequently Asked Questions. ICTR Research Navigators January 19, 2017 Version 7.
 Accelerated Translational Incubator Pilot (ATIP) Program Frequently Asked Questions ICTR Research Navigators January 19, 2017 Version 7.0 TABLE OF CONTENTS Section # Title Page 1. ABOUT THE ATIP PROGRAM...
Accelerated Translational Incubator Pilot (ATIP) Program Frequently Asked Questions ICTR Research Navigators January 19, 2017 Version 7.0 TABLE OF CONTENTS Section # Title Page 1. ABOUT THE ATIP PROGRAM...
CLOSEOUT - DON T SHUT DOWN SRA 2018 NORTHEAST/WESTERN SECTION MEETING MARCH 2018
 CLOSEOUT - DON T SHUT DOWN SRA 2018 NORTHEAST/WESTERN SECTION MEETING MARCH 2018 PRESENTATION TEAM Christine Toalepai, University of Maryland, Baltimore, Office of Sponsored Programs Administration, Sponsored
CLOSEOUT - DON T SHUT DOWN SRA 2018 NORTHEAST/WESTERN SECTION MEETING MARCH 2018 PRESENTATION TEAM Christine Toalepai, University of Maryland, Baltimore, Office of Sponsored Programs Administration, Sponsored
ORA Closeout Process for NIH Awards
 Office of Research Administration ORA Closeout Process for NIH Awards ORA CLOSEOUT GUIDELINES ORA is responsible for making sure that necessary closeout documents are submitted to NIH within 90 days of
Office of Research Administration ORA Closeout Process for NIH Awards ORA CLOSEOUT GUIDELINES ORA is responsible for making sure that necessary closeout documents are submitted to NIH within 90 days of
OMB Uniform Guidance ( UG ) Briefing. ASRSP & OSR Brown Bag Tuesday, January 27 th
 OMB Uniform Guidance ( UG ) Briefing ASRSP & OSR Brown Bag Tuesday, January 27 th Background The UG is the single biggest regulatory change in the last fifty years in research administration Interesting
OMB Uniform Guidance ( UG ) Briefing ASRSP & OSR Brown Bag Tuesday, January 27 th Background The UG is the single biggest regulatory change in the last fifty years in research administration Interesting
The Rollout of OMB A-81 and its Effect on UH
 The Rollout of OMB A-81 and its Effect on UH Cris Milligan, Interim Assistant Vice President for Research Administration Beverly Rymer, Executive Director, Office of Contracts and GrantsOffice of Contracts
The Rollout of OMB A-81 and its Effect on UH Cris Milligan, Interim Assistant Vice President for Research Administration Beverly Rymer, Executive Director, Office of Contracts and GrantsOffice of Contracts
FDP Subaward Forms Frequently Asked Questions Check back frequently for updates!
 FDP Subaward Forms Frequently Asked Questions Check back frequently for updates! Categories of Questions (click hyperlink below): Invoicing & Final Statement of Cumulative Costs Uniform Guidance (UG) data
FDP Subaward Forms Frequently Asked Questions Check back frequently for updates! Categories of Questions (click hyperlink below): Invoicing & Final Statement of Cumulative Costs Uniform Guidance (UG) data
UPDATES. Meet the Proposal Deadline. NIH: Public Access to Research Results OFFICE OF SPONSORED INSIDE THIS ISSUE:
 UPDATES INSIDE THIS ISSUE: Meet the Proposal Deadline Responsible Conduct of Research New Federal Regulations on Conflict of Interest NSF s New Proposal Guidelines and Project Reporting Changes Rutgers
UPDATES INSIDE THIS ISSUE: Meet the Proposal Deadline Responsible Conduct of Research New Federal Regulations on Conflict of Interest NSF s New Proposal Guidelines and Project Reporting Changes Rutgers
Grant Review and Submission Standard Operating Procedure
 Grant Review and Submission Standard Operating Procedure Stephen Hunt, MPA Assistant Director, Policy, Compliance & Reporting Standard Operating Procedures GRANT LIFECYCLE PRIMARY BUSINESS FUNCTIONS Application
Grant Review and Submission Standard Operating Procedure Stephen Hunt, MPA Assistant Director, Policy, Compliance & Reporting Standard Operating Procedures GRANT LIFECYCLE PRIMARY BUSINESS FUNCTIONS Application
Project Update Yoon Lee, EFM Asst Dir/PAMS Project Director Katie Cadle, PAMS Business Analyst Jessica Carney, PAMS Project Manager
 Project Update Yoon Lee, EFM Asst Dir/PAMS Project Director Katie Cadle, PAMS Business Analyst Jessica Carney, PAMS Project Manager 8/08/2013 RAF: Post Award Management System 1 Agenda What is PAMS? PAMS
Project Update Yoon Lee, EFM Asst Dir/PAMS Project Director Katie Cadle, PAMS Business Analyst Jessica Carney, PAMS Project Manager 8/08/2013 RAF: Post Award Management System 1 Agenda What is PAMS? PAMS
Sponsored Program Administration. October 10, 2017
 Sponsored Program Administration October 10, 2017 Agenda Procurement Services FDP Updates Jaggaer Pre-Award Federal Agency Updates Grants.gov Transition to Workspace NIH FORMS-E Post-Award GONE Act Certificate
Sponsored Program Administration October 10, 2017 Agenda Procurement Services FDP Updates Jaggaer Pre-Award Federal Agency Updates Grants.gov Transition to Workspace NIH FORMS-E Post-Award GONE Act Certificate
What You Need to Know When Submitting an NIH SF424 R&R Grant Application Through the UCLA Office of Contract and Grant Administration (OCGA)
 What You Need to Know When Submitting an NIH SF424 R&R Grant Application Through the UCLA Office of Contract and Grant Administration (OCGA) Kim Duiker, Assistant Director Mary Haskins, Contract & Grant
What You Need to Know When Submitting an NIH SF424 R&R Grant Application Through the UCLA Office of Contract and Grant Administration (OCGA) Kim Duiker, Assistant Director Mary Haskins, Contract & Grant
Rebecca Trahan. Office of Sponsored Programs December 9, ORED Limited Submission Update
 Rebecca Trahan Office of Sponsored Programs December 9, 2015 1 NSF Updates NIH Updates Uniform Guidance Updates and Reminders ORED Limited Submission Update OSP Updates Q & A 2 1 3 4 2 NSF 16-1 effective
Rebecca Trahan Office of Sponsored Programs December 9, 2015 1 NSF Updates NIH Updates Uniform Guidance Updates and Reminders ORED Limited Submission Update OSP Updates Q & A 2 1 3 4 2 NSF 16-1 effective
October electronic Research Administration (era) OER, OD, National Institutes of Health
 October 2016 electronic Research Administration (era) OER, OD, National Institutes of Health Scarlett Gibb era Customer Relationship Manager, era Commons Joe Schumaker era Communications and Outreach 2
October 2016 electronic Research Administration (era) OER, OD, National Institutes of Health Scarlett Gibb era Customer Relationship Manager, era Commons Joe Schumaker era Communications and Outreach 2
Fiscal Compliance Training Series: Charging Salaries Travel Expenses
 Fiscal Compliance Training Series: Charging Salaries Travel Expenses Wed., April 26, 2017, 2:00 pm to 3:00 pm Curry Student Center, Room No. 318-320-322 Fiscal Management Lifecycle 2 Conduct Research and
Fiscal Compliance Training Series: Charging Salaries Travel Expenses Wed., April 26, 2017, 2:00 pm to 3:00 pm Curry Student Center, Room No. 318-320-322 Fiscal Management Lifecycle 2 Conduct Research and
ORA Quarterly Meeting October 26, 2016 (Medical) October 28, 2016 (Coral Gables)
 ORA Quarterly Meeting October 26, 2016 (Medical) October 28, 2016 (Coral Gables) Agenda Quarterly Updates New Reporting Functionality in RRS (PI Portal) Workday Update ORA Website Presentation PCRF-Updates
ORA Quarterly Meeting October 26, 2016 (Medical) October 28, 2016 (Coral Gables) Agenda Quarterly Updates New Reporting Functionality in RRS (PI Portal) Workday Update ORA Website Presentation PCRF-Updates
December 2015 Research Administration Working Group WELCOME OFFICE OF THE VICE PRESIDENT FOR RESEARCH
 December 2015 Research Administration Working Group WELCOME Open Mike Fringe Rates FY17 proposed Budget v. Charging Distribution of Indirects PI award letter out & accounts funded Departments- this month
December 2015 Research Administration Working Group WELCOME Open Mike Fringe Rates FY17 proposed Budget v. Charging Distribution of Indirects PI award letter out & accounts funded Departments- this month
Ball State University
 Ball State University CONTRACTS AND GRANTS MANUAL 1 - Introduction... 3 1.1 Purpose of the CGO Manual... 3 1.2 Roles and Responsibilities... 3 A. Responsibilities of the Contracts and Grants Office...
Ball State University CONTRACTS AND GRANTS MANUAL 1 - Introduction... 3 1.1 Purpose of the CGO Manual... 3 1.2 Roles and Responsibilities... 3 A. Responsibilities of the Contracts and Grants Office...
UNIFORM GUIDANCE IMPLEMENTATION
 UNIFORM GUIDANCE IMPLEMENTATION Presented by Sara Judd, OSR Consultant October 2014 Uniform Guidance Implementation what we know and what we re guessing Ready, Set, Go 2014 Fred Hutchinson Cancer Research
UNIFORM GUIDANCE IMPLEMENTATION Presented by Sara Judd, OSR Consultant October 2014 Uniform Guidance Implementation what we know and what we re guessing Ready, Set, Go 2014 Fred Hutchinson Cancer Research
Proposal Development No: Date Due to Sponsor: Target Review by date: Date Review Completed:
 COEUS NIH-GRANTS.GOV PROPOSAL REVIEW CHECKLIST REVIEWER: Proposal Development No: Date Due to Sponsor: Target Review by date: Date Review Completed: Has the Program Announcement been reviewed? What is
COEUS NIH-GRANTS.GOV PROPOSAL REVIEW CHECKLIST REVIEWER: Proposal Development No: Date Due to Sponsor: Target Review by date: Date Review Completed: Has the Program Announcement been reviewed? What is
The Uniform Guidance (2 CFR, Part 200)
 WCMC Implementation of The Uniform Guidance (2 CFR, Part 200) Tuesday, September 22, 2015 & Wednesday, September 23, 2015 UG Workshop Michelle A. Lewis, M.S. Director of Research Administration Interim
WCMC Implementation of The Uniform Guidance (2 CFR, Part 200) Tuesday, September 22, 2015 & Wednesday, September 23, 2015 UG Workshop Michelle A. Lewis, M.S. Director of Research Administration Interim
Division of Grants Compliance and Oversight Office of Policy for Extramural Research Administration, OER National Institutes of Health, DHHS
 Division of Grants Compliance and Oversight Office of Policy for Extramural Research Administration, OER National Institutes of Health, DHHS NIH Regional Seminar Chicago, IL October 2016 Diane Dean, Director
Division of Grants Compliance and Oversight Office of Policy for Extramural Research Administration, OER National Institutes of Health, DHHS NIH Regional Seminar Chicago, IL October 2016 Diane Dean, Director
Sponsored Projects: Curriculum Plan Johns Hopkins University Panel Members:
 Sponsored Projects: Curriculum Plan Johns Hopkins University Panel Members: Mike Alexander, University Administration Jim Aumiller, University Administration Holly Benze, Engineering Don Cornely, University
Sponsored Projects: Curriculum Plan Johns Hopkins University Panel Members: Mike Alexander, University Administration Jim Aumiller, University Administration Holly Benze, Engineering Don Cornely, University
Children s Discovery Institute Grants Policies
 Table of Contents Section Page #(s) Purpose of Grants 2 Funding Mechanisms 2-3 Grant Identifiers 3 Authority for Making Grants 3 Amendment of Policies 3 Direct Costs 3-4 Facilities & Administrative Costs
Table of Contents Section Page #(s) Purpose of Grants 2 Funding Mechanisms 2-3 Grant Identifiers 3 Authority for Making Grants 3 Amendment of Policies 3 Direct Costs 3-4 Facilities & Administrative Costs
REQUEST FOR PROPOSALS THE ROSE HILLS FOUNDATION INNOVATOR GRANT PROGRAM RESEARCH FELLOWSHIP APPLICATION
 REQUEST FOR PROPOSALS THE ROSE HILLS FOUNDATION INNOVATOR GRANT PROGRAM RESEARCH FELLOWSHIP APPLICATION APPLICATION DEADLINE: 12:00 pm., Monday, January 9, 2017 PURPOSE The Rose Hills Foundation is a legacy
REQUEST FOR PROPOSALS THE ROSE HILLS FOUNDATION INNOVATOR GRANT PROGRAM RESEARCH FELLOWSHIP APPLICATION APPLICATION DEADLINE: 12:00 pm., Monday, January 9, 2017 PURPOSE The Rose Hills Foundation is a legacy
Ramp up and Wrap up projects. Riding the Wave to Close Out
 Ramp up and Wrap up projects Riding the Wave to Close Out To maximize our time together today: Please turn off or silence your cell phones Limit side chatter so everyone can hear Best of all. Stay for
Ramp up and Wrap up projects Riding the Wave to Close Out To maximize our time together today: Please turn off or silence your cell phones Limit side chatter so everyone can hear Best of all. Stay for
Grant/Sponsor Related Systems. Department and OSP Perspectives on ERA
 Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
Policy and Compliance: Working Together Like Hand in Glove
 Policy and Compliance: Working Together Like Hand in Glove Samuel Ashe, Director, Division of Grants Policy, OPERA, OER Diane W. Dean, Director, Division of Grants Compliance and Oversight, OPERA, OER
Policy and Compliance: Working Together Like Hand in Glove Samuel Ashe, Director, Division of Grants Policy, OPERA, OER Diane W. Dean, Director, Division of Grants Compliance and Oversight, OPERA, OER
REQUEST FOR PROPOSALS JAMES H. ZUMBERGE FACULTY RESEARCH & INNOVATION FUND ZUMBERGE INDIVIDUAL RESEARCH AWARD
 REQUEST FOR PROPOSALS JAMES H. ZUMBERGE FACULTY RESEARCH & INNOVATION FUND ZUMBERGE INDIVIDUAL RESEARCH AWARD APPLICATION DEADLINE: 5 pm, Monday, January 8, 2018 PURPOSE The primary purpose of the Zumberge
REQUEST FOR PROPOSALS JAMES H. ZUMBERGE FACULTY RESEARCH & INNOVATION FUND ZUMBERGE INDIVIDUAL RESEARCH AWARD APPLICATION DEADLINE: 5 pm, Monday, January 8, 2018 PURPOSE The primary purpose of the Zumberge
IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/ :19 PM
 IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/2018 12:19 PM Topics Covered Class Objective:. To become more familiar with the IRES
IRES Proposal Tracking (PT) Presented by: Kathi Goodfriend Office of Sponsored Projects Revised 03/15/2018 PRN: 5/14/2018 12:19 PM Topics Covered Class Objective:. To become more familiar with the IRES
UC Davis Policy and Procedure Manual
 UC Davis Policy and Procedure Manual Chapter 230, Sponsored Programs Section 07, Public Health Service Regulations on Objectivity in Research Date: Supersedes: 8/24/12 Responsible Department: Office of
UC Davis Policy and Procedure Manual Chapter 230, Sponsored Programs Section 07, Public Health Service Regulations on Objectivity in Research Date: Supersedes: 8/24/12 Responsible Department: Office of
UNIVERSITY RESEARCH ADMINISTRATION FINANCIAL ROLES AND RESPONSIBILITIES MATRIX - WORK IN PROGRESS 10/03/2013 Roles.
 UNIVERSITY RESEARCH ADMINISTRATION Roles Business Internal Controller's Clinical Responsibilities PI Office Chair Dean Audit Office OCR GCFA GCA PROVOST Trials Office I. GENERAL RESEARCH ADMINISTRATION
UNIVERSITY RESEARCH ADMINISTRATION Roles Business Internal Controller's Clinical Responsibilities PI Office Chair Dean Audit Office OCR GCFA GCA PROVOST Trials Office I. GENERAL RESEARCH ADMINISTRATION
Research Proposals & Awards Fiscal Year 2015 July 1, 2014 to June 30, 2015
 Research Proposals & Awards Fiscal Year 2015 July 1, 2014 to June 30, 2015 Report Produced December 2015 Additional ORA Resources are available at http://portal.research.ucla.edu/ Nebula Messier 78. Courtesy
Research Proposals & Awards Fiscal Year 2015 July 1, 2014 to June 30, 2015 Report Produced December 2015 Additional ORA Resources are available at http://portal.research.ucla.edu/ Nebula Messier 78. Courtesy
USF HRPP Updates October 2017
 USF HRPP Updates October 2017 Julie Moore, J.D., M.S. PA, CIP Associate Director, Research Integrity & Compliance Research Integrity and Compliance Revised Common Rule 45 CFR 46 Effective date and compliance
USF HRPP Updates October 2017 Julie Moore, J.D., M.S. PA, CIP Associate Director, Research Integrity & Compliance Research Integrity and Compliance Revised Common Rule 45 CFR 46 Effective date and compliance
(Insert additional Principal Investigators in the Comments section.) Co-Investigator Data Investigators Employee # School
 University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
Uniform Guidance. Overview and Implementation Plan. November 21, 2014
 Uniform Guidance Overview and Implementation Plan November 21, 2014 Uniform Guidance. Change is coming! College or Department name here 1 Overarching goal of the reform is to: streamline the rules and
Uniform Guidance Overview and Implementation Plan November 21, 2014 Uniform Guidance. Change is coming! College or Department name here 1 Overarching goal of the reform is to: streamline the rules and
Guidance on Effort Reporting and Certification Policies
 1. Title 2. Policy Guidance on Effort Reporting and Certification Policies Sec. 1 Sec. 2 Sec. 3 Sec. 4 Purpose. The purpose of this Policy is to identify the fundamentals of The University of Texas System
1. Title 2. Policy Guidance on Effort Reporting and Certification Policies Sec. 1 Sec. 2 Sec. 3 Sec. 4 Purpose. The purpose of this Policy is to identify the fundamentals of The University of Texas System
Federal Demonstration Partnership, NIH Updates, & Grants/Cooperative Agreement Reminders November 13, 2015
 Federal Demonstration Partnership, NIH Updates, & Grants/Cooperative Agreement Reminders November 13, 2015 Federal Demonstration Partnership BACKGROUND 154 participating institutions Goal: Improve Government
Federal Demonstration Partnership, NIH Updates, & Grants/Cooperative Agreement Reminders November 13, 2015 Federal Demonstration Partnership BACKGROUND 154 participating institutions Goal: Improve Government
The Lifecycle of a Sponsored Project
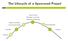 The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
University of Colorado Denver
 University of Colorado Denver Campus Guidelines Title:, 4-13 Source: Prepared by: Approved by: Office of Grants and Contracts Director, Office of Grants and Contracts Vice Chancellor for Research Effective
University of Colorado Denver Campus Guidelines Title:, 4-13 Source: Prepared by: Approved by: Office of Grants and Contracts Director, Office of Grants and Contracts Vice Chancellor for Research Effective
Presentation 2/26/2013. Director, TitleOPERA
 Presentation Title Michelle Presented G. Bulls By: Name Director, TitleOPERA Office OER AASCU Washington Conference February 21, 2013 2 NIH is operating under a continuing resolution. See NOT-OD- 13-018
Presentation Title Michelle Presented G. Bulls By: Name Director, TitleOPERA Office OER AASCU Washington Conference February 21, 2013 2 NIH is operating under a continuing resolution. See NOT-OD- 13-018
Financial Research Compliance. April 2013
 Financial Research Compliance April 2013 Overview I. What is a Sponsored Award? II. Sponsored Awards at WFU III. Compliance IV. Hot Topics V. Audits VI. WFU Updates VII. Questions VIII. Contacts What is
Financial Research Compliance April 2013 Overview I. What is a Sponsored Award? II. Sponsored Awards at WFU III. Compliance IV. Hot Topics V. Audits VI. WFU Updates VII. Questions VIII. Contacts What is
Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP)
 Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP) TITLE: Complex Award Management Program Projects/Multi-Fund Awards- Post Award NUMBER: RAS SOP 2001 VERSION: 4.0
Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP) TITLE: Complex Award Management Program Projects/Multi-Fund Awards- Post Award NUMBER: RAS SOP 2001 VERSION: 4.0
National Science Foundation Fall Grants Conference Pittsburgh, PA - November 14 & 15 - Carnegie Mellon University
 Merit Review Process National Science Foundation Fall Grants Conference Pittsburgh, PA - November 14 & 15 - Carnegie Mellon University Panelists Hao Ling Program Director, Directorate for Engineering;
Merit Review Process National Science Foundation Fall Grants Conference Pittsburgh, PA - November 14 & 15 - Carnegie Mellon University Panelists Hao Ling Program Director, Directorate for Engineering;
Georgia Institute of Technology/Georgia Tech Research Corporation A-133 Coordinated Audit Research and Development Cluster Summary Schedule of Prior
 Summary Schedule of Findings, Recommendations and Questioned Costs Status of FY 2004 Prior Year Findings and Questioned Costs Current Year Department of Audits of the State of Georgia Page FINANCIAL STATEMENT
Summary Schedule of Findings, Recommendations and Questioned Costs Status of FY 2004 Prior Year Findings and Questioned Costs Current Year Department of Audits of the State of Georgia Page FINANCIAL STATEMENT
Grants Management Workshop. Proposal and Award Management Support
 Grants Management Workshop Proposal and Award Management Support Agenda Sponsored Project Administration UVM and RSENR Support Systems Provide an overview of the grant life cycle For each stage of the
Grants Management Workshop Proposal and Award Management Support Agenda Sponsored Project Administration UVM and RSENR Support Systems Provide an overview of the grant life cycle For each stage of the
Office of Sponsored Programs Policies and Procedures. Sponsored Program Award Management Guidance
 Office of Sponsored Programs Policies and Procedures Contents Principal Investigator Eligibility... 2 Policy on Submission Deadlines for Applications Submitted by the Office of Sponsored Programs... 3
Office of Sponsored Programs Policies and Procedures Contents Principal Investigator Eligibility... 2 Policy on Submission Deadlines for Applications Submitted by the Office of Sponsored Programs... 3
Presentation. Title. Presented Name. Office of Policy for Extramural Research Administration, OER, NIH. Title. Office
 Presentation Title Michelle Presented G. Bulls, By: Director Name Office of Policy for Extramural Research Administration, OER, NIH Title Office Federal Demonstration Partnership, January 2015 NIH is currently
Presentation Title Michelle Presented G. Bulls, By: Director Name Office of Policy for Extramural Research Administration, OER, NIH Title Office Federal Demonstration Partnership, January 2015 NIH is currently
Cost Sharing Policy. Background, Scope and Purpose. Policy. Federal Guidance on Cost Sharing
 Cost Sharing Policy Background, Scope and Purpose This policy and procedure was developed to: (1) provide guidance on the circumstances in which the University may approve cost sharing commitments and
Cost Sharing Policy Background, Scope and Purpose This policy and procedure was developed to: (1) provide guidance on the circumstances in which the University may approve cost sharing commitments and
Grants Financial Procedures (Post-Award) v. 2.0
 Grants Financial Procedures (Post-Award) v. 2.0 1 Grants Financial Procedures (Post Award) Version Number: 2.0 Procedures Identifier: Superseded Procedure(s): BU-PR0001 N/A Date Approved: 9/1/2013 Effective
Grants Financial Procedures (Post-Award) v. 2.0 1 Grants Financial Procedures (Post Award) Version Number: 2.0 Procedures Identifier: Superseded Procedure(s): BU-PR0001 N/A Date Approved: 9/1/2013 Effective
Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP)
 Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP) TITLE: Research Proposal Application Process NUMBER: RAS SOP 1002 VERSION: 4.0 LAST REVISED: PREPARED BY: Office
Emory University Research Administration Services (RAS) Standard Operating Procedure (SOP) TITLE: Research Proposal Application Process NUMBER: RAS SOP 1002 VERSION: 4.0 LAST REVISED: PREPARED BY: Office
Emory Research A to Z ERAZ
 Emory Research A to Z ERAZ July 17, 2014 Whitehead Auditorium G01 Whitehead Building Agenda Controlled Substances Sub Award Processing RAS Updates SAM Kiosk Federal Updates OSP/OGCA Training Updates Christine
Emory Research A to Z ERAZ July 17, 2014 Whitehead Auditorium G01 Whitehead Building Agenda Controlled Substances Sub Award Processing RAS Updates SAM Kiosk Federal Updates OSP/OGCA Training Updates Christine
Office of Internal Audit
 Office of Internal Audit July 5, 2017 Dr. Kirk A. Calhoun, President UT Health Northeast 11937 U. S. Hwy 271 Tyler, TX 75708 Dr. Calhoun, We have completed the that was part of our Audit Plan. The objective
Office of Internal Audit July 5, 2017 Dr. Kirk A. Calhoun, President UT Health Northeast 11937 U. S. Hwy 271 Tyler, TX 75708 Dr. Calhoun, We have completed the that was part of our Audit Plan. The objective
Federal Demonstration Partnership May 13, 2013
 Current News From NIH Michelle Bulls Director Office of Policy for Extramural Research Administration Federal Demonstration Partnership May 13, 2013 1 NIH Fiscal Policy for Grant FY 2013 Consolidated and
Current News From NIH Michelle Bulls Director Office of Policy for Extramural Research Administration Federal Demonstration Partnership May 13, 2013 1 NIH Fiscal Policy for Grant FY 2013 Consolidated and
Research Administration Services Roles & Responsibilities For Grants and Contracts (Excludes Clinical Trials) Version 3.1
 Research Administration Services Roles & Responsibilities For Grants and Contracts (Excludes Clinical Trials) Version 3.1 Category PI/PI Staff Department* RAS Unit School/Unit ORA Offices Relevant SOP
Research Administration Services Roles & Responsibilities For Grants and Contracts (Excludes Clinical Trials) Version 3.1 Category PI/PI Staff Department* RAS Unit School/Unit ORA Offices Relevant SOP
Effort Certifications
 Effort Certifications Presented by Terry Shoebotham 1/12/09 prepared by Terry Shoebotham Business Management Specialist With assistance from: Contracts and Grants Accounting Main Campus and HSC TABLE OF
Effort Certifications Presented by Terry Shoebotham 1/12/09 prepared by Terry Shoebotham Business Management Specialist With assistance from: Contracts and Grants Accounting Main Campus and HSC TABLE OF
Presentation. Title. Presented By: Name. Title
 Presentation Title Presented By: Name Title Lisa Moeller, CRA, Grants Administration OfficeBranch, National Institute of General Medical Sciences, National Institutes of Health Why? Protected time for
Presentation Title Presented By: Name Title Lisa Moeller, CRA, Grants Administration OfficeBranch, National Institute of General Medical Sciences, National Institutes of Health Why? Protected time for
Finishing Your TAACCCT Grant Successfully
 Finishing Your TAACCCT Grant Successfully Understanding Grant Closeout TAACCCT On! Tuesday, September 30, 2014 Agenda Overview of the Process Final Progress Reports Submitting Grant Products and Deliverables
Finishing Your TAACCCT Grant Successfully Understanding Grant Closeout TAACCCT On! Tuesday, September 30, 2014 Agenda Overview of the Process Final Progress Reports Submitting Grant Products and Deliverables
Presentation. Title. Presented Name. Office of Policy for Extramural Research Administration, OER, NIH. Title. Office
 Presentation Title Michelle Presented G. Bulls, By: Director Name Office of Policy for Extramural Research Administration, OER, NIH Title Office Federal Demonstration Partnership, May 2015 NIH is currently
Presentation Title Michelle Presented G. Bulls, By: Director Name Office of Policy for Extramural Research Administration, OER, NIH Title Office Federal Demonstration Partnership, May 2015 NIH is currently
Financial Oversight of Sponsored Projects Principal Investigator and Department Administrator Responsibilities
 Principal Investigator and Department Administrator Responsibilities Boston College Office for Sponsored Programs Office for Research Compliance and Intellectual Property March 2004 Introduction This guide
Principal Investigator and Department Administrator Responsibilities Boston College Office for Sponsored Programs Office for Research Compliance and Intellectual Property March 2004 Introduction This guide
Policy on Principal Investigators Duties and Responsibilities on Sponsored Projects
 Office of Research and Sponsored Programs Foundation Administration Policy on Principal Investigators Duties and Responsibilities on Sponsored Projects Policy Index I. Introduction II. Policy Statement
Office of Research and Sponsored Programs Foundation Administration Policy on Principal Investigators Duties and Responsibilities on Sponsored Projects Policy Index I. Introduction II. Policy Statement
Grant and Contract Accounting
 University of Washington Faculty Grants Management Program Grant and Contract Accounting Sue Camber, Assistant Vice President Research Accounting and Analysis Grant and Contract Accounting University of
University of Washington Faculty Grants Management Program Grant and Contract Accounting Sue Camber, Assistant Vice President Research Accounting and Analysis Grant and Contract Accounting University of
Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards. AUSPAN Martha Taylor
 Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards AUSPAN 2 16 2015 Martha Taylor Grants Reform February 2011 President directed OMB to reduce unnecessary regulatory
Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards AUSPAN 2 16 2015 Martha Taylor Grants Reform February 2011 President directed OMB to reduce unnecessary regulatory
Sponsored Projects Manual
 Sponsored Projects Manual University Policies and Procedures for Requesting, Accepting, and Administering Grants, Contracts, and Cooperative Agreements February 2, 2005 CONTENTS 1. Overview... 4 1.1. Purpose
Sponsored Projects Manual University Policies and Procedures for Requesting, Accepting, and Administering Grants, Contracts, and Cooperative Agreements February 2, 2005 CONTENTS 1. Overview... 4 1.1. Purpose
Any observations not included in this report were discussed with your staff at the informal exit conference and may be subject to follow-up.
 Larry Mandel Vice Chancellor and Chief Audit Officer Office of Audit and Advisory Services 401 Golden Shore, 4th Floor Long Beach, CA 90802-4210 562-951-4430 562-951-4955 (Fax) lmandel@calstate.edu September
Larry Mandel Vice Chancellor and Chief Audit Officer Office of Audit and Advisory Services 401 Golden Shore, 4th Floor Long Beach, CA 90802-4210 562-951-4430 562-951-4955 (Fax) lmandel@calstate.edu September
PMS Project Closeout. Dan Evon, Michigan State University Nate Martinez Wayman, Duke University
 PMS Project Closeout Dan Evon, Michigan State University Nate Martinez Wayman, Duke University 1 Session Outline GAO Audit Reports Closeout Parameters The FCTR Closeout History Sample Numbers Details Late
PMS Project Closeout Dan Evon, Michigan State University Nate Martinez Wayman, Duke University 1 Session Outline GAO Audit Reports Closeout Parameters The FCTR Closeout History Sample Numbers Details Late
Developing Proposal Budgets
 Grant Writing & Developing Proposal Budgets Sandra H. Harpole Associate VP for Research Sandy Williamson ORED Fiscal Officer January 8, 2008 Idea First or Opportunity First? Idea Have an idea for a project
Grant Writing & Developing Proposal Budgets Sandra H. Harpole Associate VP for Research Sandy Williamson ORED Fiscal Officer January 8, 2008 Idea First or Opportunity First? Idea Have an idea for a project
2019 AANS Annual Scientific Meeting Abstract Instructions
 Visit MyAANS and login. Login Enter in your user ID and password. If you forgot your user ID and/or password, please use the Login Help link. Do not create another account if you cannot remember your password.
Visit MyAANS and login. Login Enter in your user ID and password. If you forgot your user ID and/or password, please use the Login Help link. Do not create another account if you cannot remember your password.
