Orientation to Research & Sponsored Projects Session II: Preparing Your Proposal. September 15, 2010
|
|
|
- Megan Walker
- 5 years ago
- Views:
Transcription
1 Orientation to Research & Sponsored Projects Session II: Preparing Your Proposal September 15,
2 Welcome & Introductions Agenda Ann Q. Gates, Associate Vice President for Research Overview: Roles & Responsibilities Proposal Development Staff Resources Creating a Network Developing a Timeline Proposal Preparation Developing a Proposal Budget IRB: Human Subjects in Research Intellectual Property Processing Your Proposal
3 Overview: Roles & Responsibilities Role of the Principal Investigator (PI) Submit the Notice of Intent Know capabilities - (knowledge & time) Meet compliance requirements Disclose conflict-of-interest, of interest, if appropriate Write proposal responsive to agency s mission & goals Obtain approvals through administrative process Submit proposal to ORSP 5 working days prior to submission Role of Research & Sponsored Projects Assist in finding & analyzing funding solicitations Assist with budget development prepare budget forms Assist with the review, edit, and provide institutional data and writing Provide final review and check solicitation requirements Prepare transmittal documents Submit the proposal to the sponsoring agency
4 PROPOSAL DEVELOPMENT
5 Proposal Development Staff Resources Funding opportunities notification Proposal development Review of solicitation guidelines & requirements Proposal Development Team: Ann Q. Gates Flo Dick Claudine M. Riccillo Malu Picard-Ami Proposal budget development Proposal submission Research Administrators: Michelle Kistenmacher Teresa Almengor Marisa Sanchez Tom Osteen Sona Kumar 5
6 Proposal Development Staff Resources Research Compliance Human subjects Biosafety-Recombinant DNA intellectual property Animal use & care Conflict of interest t certifications & assurances Athena Fester Susan Avena Cindy Crews Manuela Dokie
7 Creating a Network Activity Log on to the Expertise System (Click anywhere on image) Sign in using your user name and password Chose edit View your page Browse tabs Faculty Centers Technologies Facilities Equipment Labs & Groups
8 Developing a Proposal Timeline Plot out time from start to deadline on a calendar Break out the sections as outlined in the solicitation Determine time needed to complete each section Determine a deadline to complete each section Assign tasks if you have a team Schedule progress meetings if you have a team Use a Gantt chart, Excel, Visio or any project management sofware to develop a visual representation of your timeline
9 Proposal Preparation Outline the motivation for your work Define your project goals and hypothesis if applicable Map your goals to the program goals and ensure that there is a match Define your objectives and methods/activities Define the significance (intellectual merit) of your project Define the broader impacts Seek critical review of the major sections of your proposal from internal reviewers, ORSP Proposal Development Team, and external reviewers
10 DEVELOPING A PROPOSAL BUDGET MICHELLE KISTENMACHER, RA MANAGER TERESA ALMENGOR MARIA ISELA HERNANDEZ IRENE HOLGUIN SONA KUMAR TOM OSTEEN MARISA SANCHEZ
11 Developing a Proposal Budget A proposal is a request from an external sponsor for financial support of a research, training, equipment or outreach project The budget is the principal investigator s (PI) best estimate of the financial support needed to carry out the technical proposalp A budget that is prepared for a proposal to be submitted to a federal, state, or private agency must be consistently ss ypriced relative to proposed work and should be supported by policy, schedules, or memos regarding the costs cited
12 Developing a Proposal Budget The Proposal Budget Is a basic component of a proposal p Projects and estimates project expenses Reflects the scope of work Demonstrates investigator s capability to plan and manage a project Provides costs that are reasonable, allowable, and allocable
13 Developing a Proposal Budget The Proposal Budget Consists of the following elements: Personnel PI and Co-PIs Other Professional Students Secretary (in rare instances) Other Personnel Travel National Conferences to present research Local Mileage to perform the project
14 Developing a Proposal Budget (Continued) Participant support t Equipment Greater than $5,000 with a useful life of more than one year. Not computers Consultants Subcontracts Other direct costs Materials and supplies Publication costs Things that t don t fit anywhere else
15 Developing a Proposal Budget The ORSP Research Budget Tool
16 Activity: Using the Research Budget Tool to Develop Your Budget TASK Log on to the ORSP Website: Select Tools & Templates Select Research Budget Tool in drop-down down box Click Download: Research Budget Tool
17 IRB: HUMAN SUBJECTS S IN RESEARCH ATHENA FESTER
18 IRB: Human Subjects in Research The IRB and its Importance The Institutional Review Board (IRB) is a committee representing various research disciplines that oversees all research involving human subjects conducted by faculty, students, or staff who might use any University property or non-public information i to contact human research subjects Federal law requires that an IRB be established to review all q studies involving human subjects (both funded and unfunded protocols)
19 IRB Roles and Responsibilities To review, approve, disapprove, suspend, request modifications and/or terminate and submitted studies To process study submissions through expedited, exempt, or full board review To ensure research is being conducted in an ethical manner Ultimately-to safeguard safety for human participants y g y p p volunteering for research projects conducted by students, staff, or faculty
20 PI Roles & Responsibilities Assurance of safe and ethical study conduct by self and through supervision of study team Compliance for Human Subjects and Research training for self and all study team members Follow through on IRBNet submissions-initial, continuing review, amendments/modifications and study closure Acquisition of proper letters of collaboration to include other IRB approvals, site affiliates and letters of cooperation Proper use of IRB approved and stamped documents
21 Advisor Roles & Responsibilities To properly guide and mentor student through h the research process Ensure student is conducting and following ethical and safe research practices Ensure student has obtained proper approval to conduct research (internally and externally) Upon completion, ensure student s data is authentic and study Upon completion, ensure student s data is authentic and study has been properly closed
22 Research Submissions and Exemptions All studies utilizing data collected from human participants must be submitted for IRB review through IRBNet.org. PI s DO NOT make the determination that their study is Exempt. The IRB Chairperson or designated person will make that determination A request for Exemption may always be submitted if the study falls into one of the allotted categories Grant submissions/applications (external and internal) and IRB submissions i are two different areas Most funding agencies will want to see an IRB review determination letter (approval, exempt, deemed not research)
23 Research Submissions and Exemptions Certain activities may be exempt from IRB review because they fail to meet the definition of research: Program evaluation Service surveys Quality assurance / quality improvement Biographies or oral histories
24 Submitting Your IRB Protocol IRBNet accessed via Energizers ( Registration Creating a new study package o o o Proposal Informed Consent Study Documents (surveys, questionnaires, etc) For assistance or in-depth training, please contact Athena Fester at (915) or via at afester@utep.edu to schedule an appointment.
25 IRB: Human Subjects in Research In Closing.. Even when clear ethical standards d and principles i exist, there will be times when the need to do accurate research runs up against the rights of potential participants. p No set of standards can possibly anticipate every ethical circumstance. As you work with the IRB to resolve such issues, keep in mind the Belmont principles p of Respect for Persons, Beneficence, and Justice, and thoughtfully place yourself in the subject s position.
26 INTELLECTUAL PROPERTY INTELLECTUAL PROPERTY SUSAN AVENA
27 Intellectual Property Intellectual property (IP) is the physical embodiment of an idea Examples: research data, compounds, materials (biological, chemical), publications, presentations The Office of Technology Transfer is responsible for the management and protection of the intellectual property assets of the university Our mission is to help turn scientific discovery into tangible Our mission is to help turn scientific discovery into tangible products by evaluating disclosures for its patentability and potential commercial possibilities
28 Intellectual Property: Invention Disclosures Before the invention: Make sure you keep proper documentation Notebooks should be bound, pages numbered, entries dated, detailed descriptions of tasks per researcher, and contain data, figures, photos Important entries signed and countersigned by a witness Once the invention is made: All inventions should be reported to the Office of Technology Transfer prior to public disclosure If the invention was federally funded, the Office of Technology Transfer will report the invention to the agency and all subsequent actions relating to the invention
29 Intellectual Property: Avoid Public Disclosure Public Disclosure is: Presenting at a professional meeting Presenting to a class Publication The Law: The U.S. and Canada allow one year from the date of public disclosure to file a patent Lose the right to file in foreign countries with public disclosure Disclosure can compromise your invention and lose marketability without foreign patent rights
30 Invention Disclosure Process Submit Invention Disclosure submitted to OTT OTT will help inventor in preparing a presentation to the Intellectual Property Committee (IPC) IPC makes recommendation to VPR on whether to file a patent application CONTACTC CONTACTC Tony Woo Susan Avena Assistant Vice President for Manager Research & Technology Transfer savena@utep.edu / awoo@utep.edu / Website: h t /t
31 PROCESSING YOUR PROPOSAL
32 Processing Your Proposal: Final Steps Transmittal Form o o Signatures Attachments Final Review Electronic Submissions of Proposals o Grants.gov o NSF o NIH o DoD o DOE o US DOED o NEH o Homeland Security
33 Review: Proposal Development Process Identify the opportunity Identify team members, if applicable Submit an NOI Contact IRB, IACUC, or Tech Transfer if applicable Work with the Proposal DevT Discuss your ideas with PO Develop outline and timeline Work with RA on a budget Prepare and refine proposal Send draft for DevT and external review Secure institutional approvals Submit the final proposal
34 Contact Information & Resources ORSP Proposal Development Team ORSP Research Administrators ORSP web page Expertise System t / t /
35 Reflection What did you learn today that was new? What would you like to learn more about? What elements of the workshop did you find the most relevant?
Orientation to Research & Sponsored Projects Session I: Getting Started. September 13, 2010
 Orientation to Research & Sponsored Projects Session I: Getting Started September 13, 2010 Welcome Roberto Osegueda, Vice President for Research Introductions Ann Q. Gates, Associate Vice President for
Orientation to Research & Sponsored Projects Session I: Getting Started September 13, 2010 Welcome Roberto Osegueda, Vice President for Research Introductions Ann Q. Gates, Associate Vice President for
Minimum Proposal Requirements for Routing
 Minimum Proposal Requirements for Routing This QuickCard contains the minimum requirements for routing a proposal for approval in Coeus. Review the sponsor s solicitation for specific requirements and
Minimum Proposal Requirements for Routing This QuickCard contains the minimum requirements for routing a proposal for approval in Coeus. Review the sponsor s solicitation for specific requirements and
10 STEPS TO IRB APPROVAL
 10 STEPS TO IRB APPROVAL STEP 1: OBTAIN CITI TRAINING CERTIFICATE ALL STUDY PERSONNEL MUST TAKE AN ONLINE HUMAN SUBJECTS TRAINING EDUCATION PROGRAM. THE CERTIFICATES OF COMPLETION ARE NEEDED FOR ALL STUDY
10 STEPS TO IRB APPROVAL STEP 1: OBTAIN CITI TRAINING CERTIFICATE ALL STUDY PERSONNEL MUST TAKE AN ONLINE HUMAN SUBJECTS TRAINING EDUCATION PROGRAM. THE CERTIFICATES OF COMPLETION ARE NEEDED FOR ALL STUDY
Researcher 1: New Project Submission
 National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
Training Energizer. Researcher 1: New Project Submission. RESEARCH DATAWARE Innovation in Research Management
 IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review and oversight of research involving human subjects, animal
IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review and oversight of research involving human subjects, animal
Training Energizer. Researcher 1: New Project Submission. RESEARCH DATAWARE Innovation in Research Management
 IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review and oversight of research involving human subjects, animal
IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review and oversight of research involving human subjects, animal
Research and Economic Development at UC Riverside
 Research and Economic Development at UC Riverside Michael Pazzani Vice Chancellor for Research and Economic Development pazzani@ucr.edu http://research.ucr.edu Making an impact Overview Do great research
Research and Economic Development at UC Riverside Michael Pazzani Vice Chancellor for Research and Economic Development pazzani@ucr.edu http://research.ucr.edu Making an impact Overview Do great research
ACCOMPLISHMENTS: What was done? What was learned?
 National Science Foundation Annual Report Components (and related ATE Survey data points) REVIEW DRAFT JANAUARY 2014 NSF funded principal investigators submit annual reports to NSF via Research.gov. This
National Science Foundation Annual Report Components (and related ATE Survey data points) REVIEW DRAFT JANAUARY 2014 NSF funded principal investigators submit annual reports to NSF via Research.gov. This
Catalyst Fund Intermediate Awards Program
 Catalyst Fund Intermediate Awards Program Application deadline, November 9 th 2018 The project start date for awardees is no earlier than January 15 th, 2019 Interested applicants are welcome to seek pre-submission
Catalyst Fund Intermediate Awards Program Application deadline, November 9 th 2018 The project start date for awardees is no earlier than January 15 th, 2019 Interested applicants are welcome to seek pre-submission
CYSTIC FIBROSIS FOUNDATION
 CYSTIC FIBROSIS FOUNDATION Student Traineeship Grant POLICIES AND GUIDELINES January 5, Cystic Fibrosis Foundation 6931 Arlington Road, Suite 200 Bethesda, MD 20814 Grants and Contracts Office (301) 841-2614
CYSTIC FIBROSIS FOUNDATION Student Traineeship Grant POLICIES AND GUIDELINES January 5, Cystic Fibrosis Foundation 6931 Arlington Road, Suite 200 Bethesda, MD 20814 Grants and Contracts Office (301) 841-2614
(Insert additional Principal Investigators in the Comments section.) Co-Investigator Data Investigators Employee # School
 University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
University of Southern California Department of Contracts & Grants (DCG) Proposal Approval Record (PAR) For Dept. of Contracts & Grants Use Only USC Proposal # Date Received Deadline Date Principal Investigator
Researcher 1: New Project Submission
 National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
Research & Funding A Step-by-Step Guide
 Research & Funding A Step-by-Step Guide These steps outline what is necessary to complete a research project from start to finish. The order of the steps is required for any investigator; however, which
Research & Funding A Step-by-Step Guide These steps outline what is necessary to complete a research project from start to finish. The order of the steps is required for any investigator; however, which
Spartan RAN. Research Administrators Network Biannual Meeting April 23 rd, Before we get started, please follow the instructions on your table!
 Spartan RAN Research Administrators Network Biannual Meeting April 23 rd, 2015 Before we get started, please follow the instructions on your table! We re Going Green in 2015, so paper handouts will not
Spartan RAN Research Administrators Network Biannual Meeting April 23 rd, 2015 Before we get started, please follow the instructions on your table! We re Going Green in 2015, so paper handouts will not
Accelerated Translational Incubator Pilot (ATIP) Program. Frequently Asked Questions. ICTR Research Navigators January 19, 2017 Version 7.
 Accelerated Translational Incubator Pilot (ATIP) Program Frequently Asked Questions ICTR Research Navigators January 19, 2017 Version 7.0 TABLE OF CONTENTS Section # Title Page 1. ABOUT THE ATIP PROGRAM...
Accelerated Translational Incubator Pilot (ATIP) Program Frequently Asked Questions ICTR Research Navigators January 19, 2017 Version 7.0 TABLE OF CONTENTS Section # Title Page 1. ABOUT THE ATIP PROGRAM...
Researcher 1: New Project Submission
 National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
National Research Network Researcher 1: New Project Submission IRBNet provides the research community with an unmatched set of secure, web-based collaboration tools to support the design, management, review
IRBNet Instructions for Investigators
 IRBNet Instructions for Investigators Lifespan s Research Protection Office (RPO) uses IRBNet for the electronic administration and management of its IRB s. Below is a How to tutorial on IRBNet. Departmental
IRBNet Instructions for Investigators Lifespan s Research Protection Office (RPO) uses IRBNet for the electronic administration and management of its IRB s. Below is a How to tutorial on IRBNet. Departmental
Sponsored Programs Roles & Responsibilities
 The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
Sponsored Programs Roles & Responsibilities
 The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
The University of Florida is committed to acting with integrity in the management of sponsored programs. The goals of this document are to provide descriptions of key individuals or units and their responsibilities
IRB Process for SURF April 21, 2015
 IRB Process for SURF April 21, 2015 UNC-CH IRBs Biomedical (A,B,C,D): Expertise is focused on biomedical research (clinical trials, pharmacological research, etc) Oncology = B and D Dentistry = B and D
IRB Process for SURF April 21, 2015 UNC-CH IRBs Biomedical (A,B,C,D): Expertise is focused on biomedical research (clinical trials, pharmacological research, etc) Oncology = B and D Dentistry = B and D
MSCRF Discovery Program
 www.mscrf.org REQUEST FOR APPLICATIONS (RFA) MSCRF Discovery Program INTRODUCTION: Stem cell research offers extraordinary promise for new medical therapies and a better understanding of debilitating human
www.mscrf.org REQUEST FOR APPLICATIONS (RFA) MSCRF Discovery Program INTRODUCTION: Stem cell research offers extraordinary promise for new medical therapies and a better understanding of debilitating human
Dept/ College. Dept/ College. Dept/ College
 Office of Research Administration Matrix Revision date -2.10.17 Submitting proposals, executing awards, conducting research, and administrating sponsored projects involves many different people and units
Office of Research Administration Matrix Revision date -2.10.17 Submitting proposals, executing awards, conducting research, and administrating sponsored projects involves many different people and units
SEATTLE CHILDREN S RESEARCH INSTITUTE OPERATING POLICIES / PROCEDURES
 Financial Conflicts of Interest Page 1 of 13 SEATTLE CHILDREN S RESEARCH INSTITUTE OPERATING POLICIES / PROCEDURES DEPARTMENT: Office of Research Compliance POLICY NUMBER: ORC-003 REPLACES: RIA-03 EFFECTIVE
Financial Conflicts of Interest Page 1 of 13 SEATTLE CHILDREN S RESEARCH INSTITUTE OPERATING POLICIES / PROCEDURES DEPARTMENT: Office of Research Compliance POLICY NUMBER: ORC-003 REPLACES: RIA-03 EFFECTIVE
Demystifying the IRB
 Demystifying the IRB Mariette Marsh, MPA, CIP Assistant Director Human Subjects Protection Program Ms. Marsh is the Assistant Director for Education and Outreach at the Human Subjects Protection Program.
Demystifying the IRB Mariette Marsh, MPA, CIP Assistant Director Human Subjects Protection Program Ms. Marsh is the Assistant Director for Education and Outreach at the Human Subjects Protection Program.
Conflict of Interest Committee Submission Guide
 Conflict of Interest Committee Submission Guide Training materials for using IRBNet are available at: http://www.irbnetresources.org/ (userid: kettering, password: training) Guides and submission forms
Conflict of Interest Committee Submission Guide Training materials for using IRBNet are available at: http://www.irbnetresources.org/ (userid: kettering, password: training) Guides and submission forms
Principal Investigator User Guide
 INFOED ELECTRONIC RESEARCH ADMINISTRATION infoed.clemson.edu Principal Investigator User Guide November 2014 Version 1.7 i Document Revisions Date Version Number Document Changes 12/06/2013 1.0 Initial
INFOED ELECTRONIC RESEARCH ADMINISTRATION infoed.clemson.edu Principal Investigator User Guide November 2014 Version 1.7 i Document Revisions Date Version Number Document Changes 12/06/2013 1.0 Initial
NOVA SOUTHEASTERN UNIVERSITY
 NOVA SOUTHEASTERN UNIVERSITY DIVISION OF RESPONSIBILITIES FOR RESEARCH AND SPONSORED PROGRAMS Vice President of Research & Technology Transfer: The responsibilities of the Vice President of Research &
NOVA SOUTHEASTERN UNIVERSITY DIVISION OF RESPONSIBILITIES FOR RESEARCH AND SPONSORED PROGRAMS Vice President of Research & Technology Transfer: The responsibilities of the Vice President of Research &
MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants
 MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants RFA Release Date: Monday, February 1, 2016 Full Proposal (5 page limit) Deadline: March 16, 2016, 12:00 p.m.
MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants RFA Release Date: Monday, February 1, 2016 Full Proposal (5 page limit) Deadline: March 16, 2016, 12:00 p.m.
Subrecipient Monitoring Procedures
 Subrecipient Monitoring Procedures This procedure describes the proper management of subrecipient activity under Purdue sponsored program awards. Definitions Award: An award is a binding agreement between
Subrecipient Monitoring Procedures This procedure describes the proper management of subrecipient activity under Purdue sponsored program awards. Definitions Award: An award is a binding agreement between
MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants
 MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants RFA Release Date: Monday, April 10, 2017 Full Proposal (6 page limit) Deadline: May 16, 2017 12 p.m. (noon)
MUSC Center for Global Health Request for Applications (RFA) for Faculty Pilot Project Grants RFA Release Date: Monday, April 10, 2017 Full Proposal (6 page limit) Deadline: May 16, 2017 12 p.m. (noon)
GRANT MANAGER S HANDBOOK
 GRANT MANAGER S HANDBOOK Office of Research & Graduate Studies Foust 251 T: (989) 774-6777 F: (989) 774-3439 E: orgs@cmich.edu The Purpose of the Grant Manager s Handbook is to provide answers to commonly
GRANT MANAGER S HANDBOOK Office of Research & Graduate Studies Foust 251 T: (989) 774-6777 F: (989) 774-3439 E: orgs@cmich.edu The Purpose of the Grant Manager s Handbook is to provide answers to commonly
MENTOR-CONNECT TUTORIAL
 MENTOR-CONNECT TUTORIAL PREPARING FORMS FOR YOUR NSF ATE PROPOSAL This tutorial will guide you through an important process - filling out the forms that are required when you submit proposals to the National
MENTOR-CONNECT TUTORIAL PREPARING FORMS FOR YOUR NSF ATE PROPOSAL This tutorial will guide you through an important process - filling out the forms that are required when you submit proposals to the National
FAU RESEARCH ENHANCEMENT PROGRAMS Office of the Vice President for Research. Dissertation Year Award Application Guidelines
 FAU RESEARCH ENHANCEMENT PROGRAMS Office of the Vice President for Research Dissertation Year Award Application Guidelines OBJECTIVE OF THE GRANT To help students in their final year complete their dissertations.
FAU RESEARCH ENHANCEMENT PROGRAMS Office of the Vice President for Research Dissertation Year Award Application Guidelines OBJECTIVE OF THE GRANT To help students in their final year complete their dissertations.
Genesis Health System. Institutional Review Board. Standard Operating Procedures
 Genesis Health System Institutional Review Board Table of Contents 1. INSTITUTIONAL AUTHORITY... 6 2. PURPOSE... 6 3. THE SCOPE & AUTHORITY OF THE IRB... 7 Scope...7 Authority of the GHS-IRB...7 Authority
Genesis Health System Institutional Review Board Table of Contents 1. INSTITUTIONAL AUTHORITY... 6 2. PURPOSE... 6 3. THE SCOPE & AUTHORITY OF THE IRB... 7 Scope...7 Authority of the GHS-IRB...7 Authority
2015 TEXAS MEDICAL RESEARCH COLLABORATIVE (TEXASMRC): PROPOSAL SUBMISSION GUIDELINES
 2015 TEXAS MEDICAL RESEARCH COLLABORATIVE (TEXASMRC): PROPOSAL SUBMISSION GUIDELINES The University of Texas at Arlington (UTA), the University of Texas at Dallas (UTD) and the University of North Texas
2015 TEXAS MEDICAL RESEARCH COLLABORATIVE (TEXASMRC): PROPOSAL SUBMISSION GUIDELINES The University of Texas at Arlington (UTA), the University of Texas at Dallas (UTD) and the University of North Texas
IRB 101. Rachel Langhofer Joan Rankin Shapiro Research Administration UA College of Medicine - Phoenix
 IRB 101 Rachel Langhofer Joan Rankin Shapiro Research Administration UA College of Medicine - Phoenix Contents Brief discussion of regulations IRB Structure Levels of Approval Informed Consent HIPAA/HITECH
IRB 101 Rachel Langhofer Joan Rankin Shapiro Research Administration UA College of Medicine - Phoenix Contents Brief discussion of regulations IRB Structure Levels of Approval Informed Consent HIPAA/HITECH
DISSERTATION GRANT PROGRAM & WILLIAM SUTTLES GRADUATE FELLOWSHIP University Research Services & Administration Application Deadline: November 3, 2014
 DISSERTATION GRANT PROGRAM & WILLIAM SUTTLES GRADUATE FELLOWSHIP University Research Services & Administration Application Deadline: November 3, 2014 PURPOSE & GENERAL INFORMATION The purpose of the Dissertation
DISSERTATION GRANT PROGRAM & WILLIAM SUTTLES GRADUATE FELLOWSHIP University Research Services & Administration Application Deadline: November 3, 2014 PURPOSE & GENERAL INFORMATION The purpose of the Dissertation
Grant/Sponsor Related Systems. Department and OSP Perspectives on ERA
 Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
Grant/Sponsor Related Systems Department and OSP Perspectives on ERA Grant/Sponsor Related Systems General Proposal Submission Information ERA & Sponsor Systems National Institutes of Health BC internal
The Greenville Hospital System Office of Research Compliance and Administration HRPP Policies and Procedures
 The Greenville Hospital System Office of Research Compliance and Administration HRPP Policies and Procedures Title: Western Institutional Review Board (WIRB) HRPP Policy No. 33.02 Effective Date: May 2012
The Greenville Hospital System Office of Research Compliance and Administration HRPP Policies and Procedures Title: Western Institutional Review Board (WIRB) HRPP Policy No. 33.02 Effective Date: May 2012
Ask the Experts Panel
 Ask the Experts Panel Compliance in Research Colleen Fritsche, Assistant Director of Office of Animal Care and Use Cassie Myers, Deputy Director of Office of Human Research Ethics Chris Nelson, Director
Ask the Experts Panel Compliance in Research Colleen Fritsche, Assistant Director of Office of Animal Care and Use Cassie Myers, Deputy Director of Office of Human Research Ethics Chris Nelson, Director
ORA Closeout Process for NIH Awards
 Office of Research Administration ORA Closeout Process for NIH Awards ORA CLOSEOUT GUIDELINES ORA is responsible for making sure that necessary closeout documents are submitted to NIH within 90 days of
Office of Research Administration ORA Closeout Process for NIH Awards ORA CLOSEOUT GUIDELINES ORA is responsible for making sure that necessary closeout documents are submitted to NIH within 90 days of
Loyola University Chicago Health Sciences Division Maywood, IL. Human Subject Research Project Start-Up Guide
 Loyola University Chicago Health Sciences Division Maywood, IL Human Subject Research Project Start-Up Guide This Start-Up Guide is intended to guide you through the process of designing a research project
Loyola University Chicago Health Sciences Division Maywood, IL Human Subject Research Project Start-Up Guide This Start-Up Guide is intended to guide you through the process of designing a research project
UMCES CAYUSE 424 Training 7/21/2010 1
 UMCES CAYUSE 424 Training 7/21/2010 1 A new routing process... UMCES is moving toward using the CAYUSE424 platform for all proposal submissions. - Goal: July 1, 2010. CAYUSE is a system to system standard
UMCES CAYUSE 424 Training 7/21/2010 1 A new routing process... UMCES is moving toward using the CAYUSE424 platform for all proposal submissions. - Goal: July 1, 2010. CAYUSE is a system to system standard
POSTDOCTORAL FELLOWSHIP
 Because breast cancer is everywhere, so are we. At Susan G. Komen for the Cure, we are committed to ENDING breast cancer forever by ENERGIZING SCIENCE to find the cures and ensuring QUALITY CARE for all
Because breast cancer is everywhere, so are we. At Susan G. Komen for the Cure, we are committed to ENDING breast cancer forever by ENERGIZING SCIENCE to find the cures and ensuring QUALITY CARE for all
Research Grant Resources & Information for New Investigators
 Research Grant Resources & Information for New Investigators FEDERAL RESEARCH FUNDING 2018-2019 University of Nevada, Reno College of Engineering 1 P a g e Table of Contents A. Federal Funding Agencies.
Research Grant Resources & Information for New Investigators FEDERAL RESEARCH FUNDING 2018-2019 University of Nevada, Reno College of Engineering 1 P a g e Table of Contents A. Federal Funding Agencies.
INDIANA STATE UNIVERSITY POLICIES AND PROCEDURES FOR THE REVIEW OF RESEARCH INVOLVING HUMAN SUBJECTS
 INDIANA STATE UNIVERSITY POLICIES AND PROCEDURES FOR THE REVIEW OF RESEARCH INVOLVING HUMAN SUBJECTS This manual is believed to be in full compliance with all applicable Federal and state laws and regulations.
INDIANA STATE UNIVERSITY POLICIES AND PROCEDURES FOR THE REVIEW OF RESEARCH INVOLVING HUMAN SUBJECTS This manual is believed to be in full compliance with all applicable Federal and state laws and regulations.
HIGH POINT UNIVERSITY GRADUATE STUDENT RESEARCH AND TRAVEL FUND
 HIGH POINT UNIVERSITY GRADUATE STUDENT RESEARCH AND TRAVEL FUND The Graduate Student Research and Travel Fund was established to support graduate students engaged in scholarly research, scientific inquiry,
HIGH POINT UNIVERSITY GRADUATE STUDENT RESEARCH AND TRAVEL FUND The Graduate Student Research and Travel Fund was established to support graduate students engaged in scholarly research, scientific inquiry,
Office of Research Compliance
 Office of Research Compliance Office of Research Compliance Debra Keppler Director Responsible for providing programs to address: Responsible conduct of research training (RCR) Export control compliance
Office of Research Compliance Office of Research Compliance Debra Keppler Director Responsible for providing programs to address: Responsible conduct of research training (RCR) Export control compliance
DSRG Guidelines Doctoral Student Research Grant program Round 13
 DSRG Guidelines Doctoral Student Research Grant program Round 13 Application Period: November 1, 2017 January 31, 2018 [11:59 p.m.] Award Period: June 1, 2018 to May 31, 2019 PROGRAM DESCRIPTION The mission
DSRG Guidelines Doctoral Student Research Grant program Round 13 Application Period: November 1, 2017 January 31, 2018 [11:59 p.m.] Award Period: June 1, 2018 to May 31, 2019 PROGRAM DESCRIPTION The mission
Life Sciences Simons Collaboration on the Global Brain (SCGB) Fellowships
 Life Sciences Simons Collaboration on the Global Brain (SCGB) Fellowships Fellowship Policies and Procedures Table of Contents 1. Our mission Page 2 2. Online fellowship management Page 2 3. Fellowship
Life Sciences Simons Collaboration on the Global Brain (SCGB) Fellowships Fellowship Policies and Procedures Table of Contents 1. Our mission Page 2 2. Online fellowship management Page 2 3. Fellowship
I. Scope This policy defines unanticipated problems and adverse events and establishes the reporting process and timeline.
 Human Research Protection Program Policies & Procedures Unanticipated Problems and Adverse Events Version 3.0 Date Effective: 11.9.2012 Research Integrity Office Mail code L106-RI Portland, Oregon 97239-3098
Human Research Protection Program Policies & Procedures Unanticipated Problems and Adverse Events Version 3.0 Date Effective: 11.9.2012 Research Integrity Office Mail code L106-RI Portland, Oregon 97239-3098
Request for Proposals for Student Research
 Request for Proposals for Student Research RFP Title: Child Injury Prevention Research RFP Number: 2013 S -001 1 Introduction 1.1 About CChIPS The Center for Child Injury Prevention Studies (CChIPS) is
Request for Proposals for Student Research RFP Title: Child Injury Prevention Research RFP Number: 2013 S -001 1 Introduction 1.1 About CChIPS The Center for Child Injury Prevention Studies (CChIPS) is
Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan
 Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan TABLE OF CONTENTS GRASP COMPLIANCE PROGRAM Policy Applicability Components Administration GRASP COMPLIANCE PLAN Introduction
Grants, Research and Sponsored Programs (GRASP) Compliance Program and Plan TABLE OF CONTENTS GRASP COMPLIANCE PROGRAM Policy Applicability Components Administration GRASP COMPLIANCE PLAN Introduction
CAYUSE Research Suite
 CAYUSE Research Suite Internal Clearance & Submission Process Principal Investigator/Program Director Cayuse SP is used by ORSP to process Internal Clearance and when applicable to submit a proposal for
CAYUSE Research Suite Internal Clearance & Submission Process Principal Investigator/Program Director Cayuse SP is used by ORSP to process Internal Clearance and when applicable to submit a proposal for
YALE UNIVERSITY THE RESEARCHERS GUIDE TO HIPAA. Health Insurance Portability and Accountability Act of 1996
 YALE UNIVERSITY THE RESEARCHERS GUIDE TO HIPAA Health Insurance Portability and Accountability Act of 1996 Handbook Table of Contents I. Introduction What is HIPAA? What is PHI? What is a Covered Entity
YALE UNIVERSITY THE RESEARCHERS GUIDE TO HIPAA Health Insurance Portability and Accountability Act of 1996 Handbook Table of Contents I. Introduction What is HIPAA? What is PHI? What is a Covered Entity
MDF Request for Applications (RFA) AWARD POLICY
 MDF Request for Applications (RFA) AWARD POLICY The Myotonic Dystrophy Foundation (MDF) is the world s largest patient organization focused solely on myotonic dystrophy. Our mission, Care and a Cure, is
MDF Request for Applications (RFA) AWARD POLICY The Myotonic Dystrophy Foundation (MDF) is the world s largest patient organization focused solely on myotonic dystrophy. Our mission, Care and a Cure, is
Scott Spear Innovation in Breast Reconstruction Fellowship Funded by the Allergan Foundation
 Na Scott Spear Innovation in Breast Reconstruction Fellowship Funded by the Allergan Foundation Grant Application Guidelines and Eligibility Submission Deadline: Monday January 29, 2018 Eligibility Applicants
Na Scott Spear Innovation in Breast Reconstruction Fellowship Funded by the Allergan Foundation Grant Application Guidelines and Eligibility Submission Deadline: Monday January 29, 2018 Eligibility Applicants
Request for Proposals for Faculty Research
 Request for Proposals for Faculty Research RFP Title: Child Injury Prevention Research RFP Number 2013 F - 001 1 Introduction 1.1 About CChIPS The Center for Child Injury Prevention Studies (CChIPS) is
Request for Proposals for Faculty Research RFP Title: Child Injury Prevention Research RFP Number 2013 F - 001 1 Introduction 1.1 About CChIPS The Center for Child Injury Prevention Studies (CChIPS) is
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research
 Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director Craig O Neill, Office of Sponsored Programs, Manager February
Finding Funding, Budget Preparation, and Proposal Submission for Sponsored Research Laura Johnston, Office of Sponsored Programs, Asst. Director Craig O Neill, Office of Sponsored Programs, Manager February
The PI or their Sponsor s donation history to the PSF may also be considered in the review of the application. Preparing to Apply
 Na Research Fellowship Grant Application Guidelines and Eligibility Submission Deadline: Thursday, December 1st, 2017 Eligibility Applicants must be a MD or DO hold a full-time position in a U.S. or Canadian
Na Research Fellowship Grant Application Guidelines and Eligibility Submission Deadline: Thursday, December 1st, 2017 Eligibility Applicants must be a MD or DO hold a full-time position in a U.S. or Canadian
Grand Challenges Explorations Round 17 Rules & Guidelines Proposals due by Wednesday, May 11, 2016, 11:30 a.m. U.S. Pacific Standard Time
 Grand Challenges Explorations Round 17 Rules & Guidelines Proposals due by Wednesday, May 11, 2016, 11:30 a.m. U.S. Pacific Standard Time I. Overview Grand Challenges Explorations (GCE) supports hundreds
Grand Challenges Explorations Round 17 Rules & Guidelines Proposals due by Wednesday, May 11, 2016, 11:30 a.m. U.S. Pacific Standard Time I. Overview Grand Challenges Explorations (GCE) supports hundreds
Office of Research Integrity. CITI Program User Manual
 Office of Research Integrity CITI Program User Manual 04/11/2016 User Manual The Office of Research Integrity is pleased to provide Ball State researchers with this manual to assist them in completing
Office of Research Integrity CITI Program User Manual 04/11/2016 User Manual The Office of Research Integrity is pleased to provide Ball State researchers with this manual to assist them in completing
WHAT IS AN IRB? WHAT IS AN IRB? 3/25/2015. Presentation Outline
 Education &Training WHAT IS AN IRB? Introduction to the UofL Institutional Review Boards & Human Subjects Protection Program IRB Review Process Post Approval Monitoring March 2015 1 Presentation Outline
Education &Training WHAT IS AN IRB? Introduction to the UofL Institutional Review Boards & Human Subjects Protection Program IRB Review Process Post Approval Monitoring March 2015 1 Presentation Outline
CYSTIC FIBROSIS FOUNDATION
 CYSTIC FIBROSIS FOUNDATION Second Year Clinical Fellowship Application POLICIES AND GUIDELINES December 7, 2016 Cystic Fibrosis Foundation 6931 Arlington Road, Suite 200 Bethesda, MD 20814 Grants and Contracts
CYSTIC FIBROSIS FOUNDATION Second Year Clinical Fellowship Application POLICIES AND GUIDELINES December 7, 2016 Cystic Fibrosis Foundation 6931 Arlington Road, Suite 200 Bethesda, MD 20814 Grants and Contracts
CYSTIC FIBROSIS FOUNDATION
 CYSTIC FIBROSIS FOUNDATION Student Traineeship Award POLICIES AND GUIDELINES Published: January 24, 2018 Application (rolling) Deadline: December 31, 2018 Cystic Fibrosis Foundation 4550 Montgomery Avenue,
CYSTIC FIBROSIS FOUNDATION Student Traineeship Award POLICIES AND GUIDELINES Published: January 24, 2018 Application (rolling) Deadline: December 31, 2018 Cystic Fibrosis Foundation 4550 Montgomery Avenue,
Career Catalyst request for applications. Because breast cancer is everywhere, so are we.
 Because breast cancer is everywhere, so are we. At Susan G. Komen for the Cure, we are committed to ENDING breast cancer forever by ENERGIZING SCIENCE to find the cures and ensuring QUALITY CARE for all
Because breast cancer is everywhere, so are we. At Susan G. Komen for the Cure, we are committed to ENDING breast cancer forever by ENERGIZING SCIENCE to find the cures and ensuring QUALITY CARE for all
IRB Application the Basics GETTING STARTED
 IRB Application the Basics GETTING STARTED Is my project Human Subjects Research? Research is defined as a systematic investigation, including research development, testing and evaluation, designed to
IRB Application the Basics GETTING STARTED Is my project Human Subjects Research? Research is defined as a systematic investigation, including research development, testing and evaluation, designed to
West Virginia Clinical and Translational Science Institute Open Competition RFA
 West Virginia Clinical and Translational Science Institute Open Competition RFA Part 1. Overview Information The goal of this Request for Applications (RFA) is to support clinical and translational pilot
West Virginia Clinical and Translational Science Institute Open Competition RFA Part 1. Overview Information The goal of this Request for Applications (RFA) is to support clinical and translational pilot
DO I NEED TO SUBMIT FOR THIS?... & OTHER FREQUENTLY ASKED QUESTIONS. March 2015 IRB Forum
 DO I NEED TO SUBMIT FOR THIS?... & OTHER FREQUENTLY ASKED QUESTIONS March 2015 IRB Forum Topics Quality Assurance/Quality Improvement Projects Informed Consent- when is a waiver appropriate? Retrospective/Prospective
DO I NEED TO SUBMIT FOR THIS?... & OTHER FREQUENTLY ASKED QUESTIONS March 2015 IRB Forum Topics Quality Assurance/Quality Improvement Projects Informed Consent- when is a waiver appropriate? Retrospective/Prospective
Lions Clubs International Foundation (LCIF) SightFirst Research Grant Request for Proposals
 Lions Clubs International Foundation (LCIF) SightFirst Research Grant 2017-2018 Request for Proposals Introduction The LCIF SightFirst program strengthens eye care systems in underserved communities enabling
Lions Clubs International Foundation (LCIF) SightFirst Research Grant 2017-2018 Request for Proposals Introduction The LCIF SightFirst program strengthens eye care systems in underserved communities enabling
Life Sciences Simons Collaboration on the Origins of Life Fellowship Policies and Procedures
 Life Sciences Simons Collaboration on the Origins of Life Fellowship Policies and Procedures Table of Contents 1. Our mission Page 2 2. Online fellowship management Page 2 3. Fellowship activation Page
Life Sciences Simons Collaboration on the Origins of Life Fellowship Policies and Procedures Table of Contents 1. Our mission Page 2 2. Online fellowship management Page 2 3. Fellowship activation Page
PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR CONTACT INFORMATION Prefix: First Name: Middle Name: Last Name: Suffix:
 Proposal Summary Proposal Number Sponsor Deadline Proposal Status: Submission Method: Submission Type: Pre-application Application Changed/Corrected INVESTIGATOR DATA PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR
Proposal Summary Proposal Number Sponsor Deadline Proposal Status: Submission Method: Submission Type: Pre-application Application Changed/Corrected INVESTIGATOR DATA PROJECT DIRECTOR / PRINCIPAL INVESTIGATOR
2018 FELLOWSHIP GUIDELINES Accepting Applications May 10, 2018 June 28, 2018
 2018 FELLOWSHIP GUIDELINES Accepting Applications May 10, 2018 June 28, 2018 The Prevent Cancer Foundation is the only U.S. nonprofit organization solely devoted to cancer prevention and early detection.
2018 FELLOWSHIP GUIDELINES Accepting Applications May 10, 2018 June 28, 2018 The Prevent Cancer Foundation is the only U.S. nonprofit organization solely devoted to cancer prevention and early detection.
System to Track and Approve Research STAR Principal Investigator/Proxy User Guide
 System to Track and Approve Research STAR Principal Investigator/Proxy User Guide Research Administration 125 Worth Street, 4 th Floor New York, NY 10013 Contents I) BACKGROUND INFORMATION... 3 II) ACCESSING
System to Track and Approve Research STAR Principal Investigator/Proxy User Guide Research Administration 125 Worth Street, 4 th Floor New York, NY 10013 Contents I) BACKGROUND INFORMATION... 3 II) ACCESSING
The AOFAS Research Grants Program Description, Policies, and Guidelines for Applicants and Institutional Representatives
 The AOFAS Research Grants Program Description, Policies, and Guidelines for Applicants and Institutional Representatives Updated October 2016 for the 2017 Grants Cycle Table of Contents ABOUT THE AOFAS
The AOFAS Research Grants Program Description, Policies, and Guidelines for Applicants and Institutional Representatives Updated October 2016 for the 2017 Grants Cycle Table of Contents ABOUT THE AOFAS
The Lifecycle of a Sponsored Project
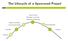 The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
The Lifecycle of a Sponsored Project Active Period Spending, Invoicing, Reporting, Monitoring Award Processing And Account Setup Final Reporting Proposal Closeout Funding Funding Sources Where is the Money?
Proposal Summary Example
 Example 04/2012 https://umces.cayuse424.com:8443/319/showproposalpage.do?subsessionid=0&url=/... Proposal Summary Proposal Number AL2011-999NSF Proposal Status: Incomplete Sponsor Deadline 01/01/2012 Submission
Example 04/2012 https://umces.cayuse424.com:8443/319/showproposalpage.do?subsessionid=0&url=/... Proposal Summary Proposal Number AL2011-999NSF Proposal Status: Incomplete Sponsor Deadline 01/01/2012 Submission
Proposal Development Guide
 Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 8 Sponsor & Program
Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 8 Sponsor & Program
CYSTIC FIBROSIS FOUNDATION
 CYSTIC FIBROSIS FOUNDATION Screening Improvement Program (SIP) for Optimizing the Diagnosis of Infants Award POLICIES AND GUIDELINES Published: January 17, 2018 Application Deadline: April 2, 2018 Cystic
CYSTIC FIBROSIS FOUNDATION Screening Improvement Program (SIP) for Optimizing the Diagnosis of Infants Award POLICIES AND GUIDELINES Published: January 17, 2018 Application Deadline: April 2, 2018 Cystic
SECNAVINST E ONR Dec 2017 SECNAV INSTRUCTION E. From: Secretary of the Navy. Subj: HUMAN RESEARCH PROTECTION PROGRAM
 ONR 343 SECNAV INSTRUCTION 3900.39E From: Secretary of the Navy Subj: HUMAN RESEARCH PROTECTION PROGRAM Encl: (1) Changes (2) References (3) Responsibilities (4) Procedures (5) Definitions (6) Reports
ONR 343 SECNAV INSTRUCTION 3900.39E From: Secretary of the Navy Subj: HUMAN RESEARCH PROTECTION PROGRAM Encl: (1) Changes (2) References (3) Responsibilities (4) Procedures (5) Definitions (6) Reports
Administrative Burden of Research Compliance
 Administrative Burden of Research Compliance Measuring and Minimizing David L. Wynes, Ph.D. Vice President for Research Administration Emory University 1 FDP Faculty Burden Survey (X2) PIs estimated that
Administrative Burden of Research Compliance Measuring and Minimizing David L. Wynes, Ph.D. Vice President for Research Administration Emory University 1 FDP Faculty Burden Survey (X2) PIs estimated that
Indiana University Health Values Fund Grant Pilot & Feasibility Program - Research
 Request for Applications Indiana University Health Values Fund Grant Pilot & Feasibility Program - Research a joint initiative between INDIANA UNIVERSITY HEALTH & INDIANA CLINICAL AND TRANSLATIONAL SCIENCES
Request for Applications Indiana University Health Values Fund Grant Pilot & Feasibility Program - Research a joint initiative between INDIANA UNIVERSITY HEALTH & INDIANA CLINICAL AND TRANSLATIONAL SCIENCES
COULTER TRANSLATIONAL RESEARCH AWARDS 2015 FULL FUNDING ROUND APPLICATION & ADMINISTRATIVE GUIDELINES
 COULTER TRANSLATIONAL RESEARCH AWARDS 2015 FULL FUNDING ROUND APPLICATION & ADMINISTRATIVE GUIDELINES The Wallace H. Coulter Foundation provides an operational roadmap to accelerate and fund the translation
COULTER TRANSLATIONAL RESEARCH AWARDS 2015 FULL FUNDING ROUND APPLICATION & ADMINISTRATIVE GUIDELINES The Wallace H. Coulter Foundation provides an operational roadmap to accelerate and fund the translation
APPLIES TO: ALL DDPSC Scientific Employees, Students, Visiting Scientists RETENTION OF AND ACCESS TO RESEARCH DATA
 APPLIES TO: ALL DDPSC Scientific Employees, Students, Visiting Scientists TITLE: RETENTION OF AND ACCESS TO RESEARCH DATA PURPOSE: Maintaining accurate and appropriate research records is an essential
APPLIES TO: ALL DDPSC Scientific Employees, Students, Visiting Scientists TITLE: RETENTION OF AND ACCESS TO RESEARCH DATA PURPOSE: Maintaining accurate and appropriate research records is an essential
ONBOARDING PROCEDURE CHECKLIST
 Northwestern RESEARCH OFFICE FOR RESEARCH INTEGRITY Research-Related PI ONBOARDING PROCEDURE CHECKLIST Before beginning this checklist you should first have a NetID. SPONSORED RESEARCH Are there awards
Northwestern RESEARCH OFFICE FOR RESEARCH INTEGRITY Research-Related PI ONBOARDING PROCEDURE CHECKLIST Before beginning this checklist you should first have a NetID. SPONSORED RESEARCH Are there awards
2018 GRANT GUIDELINES Accepting Applications May 10, 2018 June 28, 2018
 2018 GRANT GUIDELINES Accepting Applications May 10, 2018 June 28, 2018 The Prevent Cancer Foundation is the only U.S. nonprofit organization solely devoted to cancer prevention and early detection. Since
2018 GRANT GUIDELINES Accepting Applications May 10, 2018 June 28, 2018 The Prevent Cancer Foundation is the only U.S. nonprofit organization solely devoted to cancer prevention and early detection. Since
Standard Operating Procedures for P209: Investigator Conflict of Interest Policy
 Standard Operating Procedures for P209: Investigator Conflict of Interest Policy Table of Contents Applicability... 4 Institutional Roles... 5 Conflict of Interest (COI) Committee... 5 Designated Institutional
Standard Operating Procedures for P209: Investigator Conflict of Interest Policy Table of Contents Applicability... 4 Institutional Roles... 5 Conflict of Interest (COI) Committee... 5 Designated Institutional
Acquisition, Management, Sharing, and Ownership of Data
 Acquisition, Management, Sharing, and Ownership of Data Responsible Conduct of Research Training F R A N K V A N B R E U K E L E N U N I V E R S I T Y O F N E V A D A L A S V E G A S P R E S E N T E D
Acquisition, Management, Sharing, and Ownership of Data Responsible Conduct of Research Training F R A N K V A N B R E U K E L E N U N I V E R S I T Y O F N E V A D A L A S V E G A S P R E S E N T E D
Strategic Plan wmich.edu/research
 wmich.edu/research INTRODUCTION Western Michigan University is learner centered, discovery driven, and globally engaged. It became Michigan s fourth public university in 1957 and today offers 147 bachelor
wmich.edu/research INTRODUCTION Western Michigan University is learner centered, discovery driven, and globally engaged. It became Michigan s fourth public university in 1957 and today offers 147 bachelor
CCF RESEARCH GRANT APPLICATION 2017 REQUIREMENTS & GUIDELINES
 CCF RESEARCH GRANT APPLICATION 2017 REQUIREMENTS & GUIDELINES The Children s Cardiomyopathy Foundation (CCF) s Research Grant Program has a two- step application process. A letter of intent is required
CCF RESEARCH GRANT APPLICATION 2017 REQUIREMENTS & GUIDELINES The Children s Cardiomyopathy Foundation (CCF) s Research Grant Program has a two- step application process. A letter of intent is required
Grants Module. Create and Submit a Funding Proposal for Non System to System Submissions
 Grants Module Create and Submit a Funding Proposal for Non System to System Submissions Summary of Major Changes The following directions are a combination of all steps in the Funding Proposal submission
Grants Module Create and Submit a Funding Proposal for Non System to System Submissions Summary of Major Changes The following directions are a combination of all steps in the Funding Proposal submission
Frequently Asked Questions. The CIRB is located at 168 Jalan Bukit Merah #06-08 Tower 3 Connection One Singapore
 1. Where is the location of the CIRB? The CIRB is located at 168 Jalan Bukit Merah #06-08 Tower 3 Connection One Singapore 150168. 2. When is the submission deadline? The CIRB meets once a month. The submission
1. Where is the location of the CIRB? The CIRB is located at 168 Jalan Bukit Merah #06-08 Tower 3 Connection One Singapore 150168. 2. When is the submission deadline? The CIRB meets once a month. The submission
NPGH Global Health Program for Fellows and Scholars Application
 Resize font: NPGH Global Health Program for Fellows and Scholars Application 2019-2020 Thank you for your interest in the rthern Pacific Global Health Fellows and Scholars Program. Please complete the
Resize font: NPGH Global Health Program for Fellows and Scholars Application 2019-2020 Thank you for your interest in the rthern Pacific Global Health Fellows and Scholars Program. Please complete the
HIC Standard Operating Procedure. For-Cause Audits of Human Research Studies
 HIC Standard Operating Procedure For-Cause Audits of Human Research Studies Background As part of the Wayne State University (WSU) Human Investigation Committee s (HIC) Human Research Protection Program,
HIC Standard Operating Procedure For-Cause Audits of Human Research Studies Background As part of the Wayne State University (WSU) Human Investigation Committee s (HIC) Human Research Protection Program,
Roles and Responsibilities of Students and Adults
 Roles and Responsibilities of Students and Adults 1) The Student Researcher(s) The student researcher is responsible for all aspects of the research project including enlisting the aid of any needed supervisory
Roles and Responsibilities of Students and Adults 1) The Student Researcher(s) The student researcher is responsible for all aspects of the research project including enlisting the aid of any needed supervisory
Anesthesia Patient Safety Foundation
 Anesthesia Patient Safety Foundation Annual Investigator Initiated Research Grant DEADLINE February 12, 2018 (5 PM EST) This program s objective is to stimulate and fund studies that will clearly improve
Anesthesia Patient Safety Foundation Annual Investigator Initiated Research Grant DEADLINE February 12, 2018 (5 PM EST) This program s objective is to stimulate and fund studies that will clearly improve
UNC Lineberger Developmental Funding Program. Proposal Due Dates: 5:00pm March 15 and September 15
 Proposal Due Dates: 5:00pm March 15 and September 15 Letter of Intent for Tier 3: Multi-Project Awards due three weeks before due date The UNC Lineberger Developmental Funding Program is intended to support
Proposal Due Dates: 5:00pm March 15 and September 15 Letter of Intent for Tier 3: Multi-Project Awards due three weeks before due date The UNC Lineberger Developmental Funding Program is intended to support
Abstract Submission Tutorial Step-by-Step Instructions with Screen Shots. journalofvision.org tvstjournal.
 Abstract Submission Tutorial Step-by-Step Instructions with Screen Shots 1 Deadlines Friday, December 1, 11:59 pm, U.S. ET, 2017. After the December 1 deadline, the start of any draft abstracts will not
Abstract Submission Tutorial Step-by-Step Instructions with Screen Shots 1 Deadlines Friday, December 1, 11:59 pm, U.S. ET, 2017. After the December 1 deadline, the start of any draft abstracts will not
1.2.1 It is the policy of the University of Alabama that qualifying research may be reviewed using an expedited procedure.
 POLICY # RESEARCH POLICY & PROCEDURE EXPEDITED REVIEW Approval Date: 2-9-2012 CTM Review Cycle: 1 2 3 Revision Dates: AAHRPP DOC # 66 Responsible Office: Research Compliance 1.0 POLICY 1.1 Background 1.1.1
POLICY # RESEARCH POLICY & PROCEDURE EXPEDITED REVIEW Approval Date: 2-9-2012 CTM Review Cycle: 1 2 3 Revision Dates: AAHRPP DOC # 66 Responsible Office: Research Compliance 1.0 POLICY 1.1 Background 1.1.1
SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME
 SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME OVPR/SPS News and Information Technology Commercialization Services Andrew Zehner 2017 Individual Financial Disclosure in Research Internal Routing
SPONSORED PROGRAM ADMINISTRATION MEETING March 2017 WELCOME OVPR/SPS News and Information Technology Commercialization Services Andrew Zehner 2017 Individual Financial Disclosure in Research Internal Routing
